Insights into the pharmaceutical properties and in silico study of novel hydrazone derivatives
- PMID: 39622881
- PMCID: PMC11612229
- DOI: 10.1038/s41598-024-81555-z
Insights into the pharmaceutical properties and in silico study of novel hydrazone derivatives
Abstract
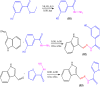
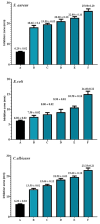
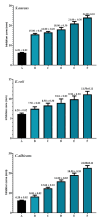
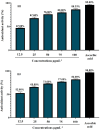
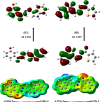
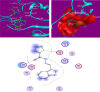
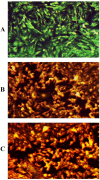
It has been established that the Hydrazone derivatives have important pharmacological effects. In the first step, hydrazine (NH2NH2) reacts with a compound containing a carbonyl group (C = O) in the presence of ethanol and heat, leading to the formation of hydrazone compound (H1). The second step is the formation of the Schiff base (H2) by the reaction of compound (H1) with indole, ethanol, and acetic acid, which contain a double bond (C = N). In the third step, the (H1) reacts with thiophene, ethanol, and acetic acid to form a compound (H3) containing multiple bonds between the indole and thiophene rings. The synthetic test compounds underwent characterization using TLC, IR, 1 H - NMR, and ¹3C NMR spectral examinations. Both compounds, H2 and H3, exhibit antioxidant activity at different concentrations (from 12.5 to 100 µgmL- 1), where the effect increases gradually with the increase in concentration. The compounds (H2 and H3) exhibited an apparent inhibitory effect on the growth of Staphylococcus aureus, Escherichia coli, and Candida albicans. Calculations have been performed for DFT, molecular docking, molecular dynamics simulations, and the ADMET protocol, and they are essential for describing the interaction and stability. In hydrazone derivatives, groups like amine, hydroxy, thiophene, and indole form hydrogen bonds and electrostatic interactions with amino acids such as arginine, lysine, glutamic acid, and aspartic acid. These interactions are crucial to evaluating the compound's stability and its potential to inhibit enzyme activity. The results indicate that the compound shows strong binding and stability at the active site, making it a promising candidate for further studies as an anti-colon cancer agent.
Keywords: Anticancer; Antimicrobial; Antioxidant; Docking study; Homo-LUMO; Hydrazone.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures



References
-
- Priya, B., Utreja, D., Sharma, S., Kaur, G. & Madhvi. Synthesis and antimicrobial activities of indole-based Schiff bases and their metal complexes: a review. Curr. Org. Chem.27, 941–961. 10.2174/1385272827666230901140611 (2023).
-
- Ibraheem, H. H., Issa, A. A. & El-Sayed, D. S. Structural behavior and surface layer modification of (E)-N’-((1H-indol-3-yl) methylene)-4-chlorobenzohydrazide: Spectroscopic, DFT, biomedical activity and molecular dynamic simulation against Candida Albicans receptor. J. Mol. Struct.1312, 138484. 10.1016/j.molstruc.2024.138484 (2024).
-
- Adel, M. H. & Ibraheem, H. Synthesis, molecular docking, and anti-tumor activity of pyrazole derivatives. In Fifth International Conference on Applied Sciences: ICAS2023. (2023)., December 10.1063/5.0210692
-
- Jima’a, R. B. & Shaalan, N. D. Synthesis, characterization, and biological activity of new metal ion complexes with Schiff base derived from 2-acetylthiophene and isatin. Egypt. J. Chem.10.21608/EJCHEM.2022.124768.5552 (2022).
-
- AL-Hashemmi, A. & Farhan, M. S. Synthesis, identification and preliminary pharmacological evaluation of new hydrazone and 1, 3, 4-oxadiazole derivatives of ketorolac. Iraqi J. Pharm. Sci.33 (1), 113–122. 10.31351/vol33iss1pp113-122 (2024).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

