Rhythmic astrocytic GABA production synchronizes neuronal circadian timekeeping in the suprachiasmatic nucleus
- PMID: 39623138
- PMCID: PMC11731042
- DOI: 10.1038/s44318-024-00324-w
Rhythmic astrocytic GABA production synchronizes neuronal circadian timekeeping in the suprachiasmatic nucleus
Abstract
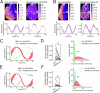
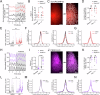
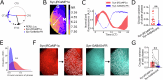
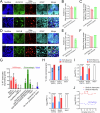
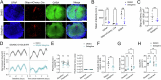
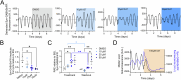
Astrocytes of the suprachiasmatic nucleus (SCN) can regulate sleep-wake cycles in mammals. However, the nature of the information provided by astrocytes to control circadian patterns of behavior is unclear. Neuronal circadian activity across the SCN is organized into spatiotemporal waves that govern seasonal adaptations and timely engagement of behavioral outputs. Here, we show that astrocytes across the mouse SCN exhibit instead a highly uniform, pulse-like nighttime activity. We find that rhythmic astrocytic GABA production via polyamine degradation provides an inhibitory nighttime tone required for SCN circuit synchrony, thereby acting as an internal astrocyte zeitgeber (or "astrozeit"). We further identify synaptic GABA and astrocytic GABA as two key players underpinning coherent spatiotemporal circadian patterns of SCN neuronal activity. In describing a new mechanism by which astrocytes contribute to circadian timekeeping, our work provides a general blueprint for understanding how astrocytes encode temporal information underlying complex behaviors in mammals.
Keywords: Astrocyte; Circadian Clock; GABA; MAO-B; SCN.
© 2024. The Author(s).
Conflict of interest statement
Disclosure and competing interests statement. The authors declare no competing interests.
Figures







References
-
- Aguilar-Roblero R, Verduzco-Carbajal L, Rodríguez C, Mendez-Franco J, Morán J, de la Mora MP (1993) Circadian rhythmicity in the GABAergic system in the suprachiasmatic nuclei of the rat. Neurosci Lett 157:199–202 - PubMed
-
- Barakat L, Bordey A (2002) GAT-1 and reversible GABA transport in Bergmann glia in slices. J Neurophysiol 88:1407–1419 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

