Low dose Adenoviral Vammin gene transfer induces myocardial angiogenesis and increases left ventricular ejection fraction in ischemic porcine heart
- PMID: 39623213
- PMCID: PMC11611887
- DOI: 10.1038/s41598-024-81773-5
Low dose Adenoviral Vammin gene transfer induces myocardial angiogenesis and increases left ventricular ejection fraction in ischemic porcine heart
Abstract
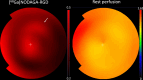
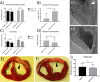
This preliminary study investigated if VEGFR-2 selective adenoviral Vammin (AdVammin) gene therapy could induce angiogenesis and increase perfusion in the healthy porcine myocardium. Also, we determined using a clinically relevant large animal model if AdVammin gene therapy could improve the function of a chronically ischemic heart. Low doses of AdVammin (dose range 2 × 109-2 × 1010 vp) gene transfers were performed into the porcine myocardium using an endovascular injection catheter. AdCMV was used as a control. The porcine model of chronic myocardial ischemia was used in the ischemic studies. The AdVammin enlarged the mean capillary area and stimulated pericyte coverage in the target area 6 days after the gene transfers. Using positron emission tomography 15O-radiowater imaging, we demonstrated that AdVammin gene therapy increased perfusion in healthy myocardium at rest. AdVammin treatment also increased ejection fraction at stress in the ischemic heart, as detected using left ventricular cine angiography. In addition, we demonstrated successful in vivo imaging of enhanced angiogenesis using [68Ga]NODAGA-RGD peptide. However, AdVammin also increased tissue permeability and was associated with significant pericardial fluid accumulation, limiting AdVammin's therapeutic potential and emphasizing the importance of correct dosage.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: NJ is a shareholder of Saparo Translational Research Oy, a CRO for large animal studies. Ethical statement: The Finnish National Animal Experiment Board and the Animal Experiment Board of the University of Finland approved all animal experiments. All animal experiments were in compliance with the ARRIVE guidelines. People involved in animal work have Felasa B or C certification.
Figures

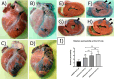
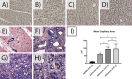
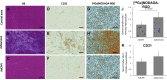
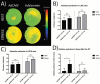
References
-
- Neumann, F. J. et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. European Heart Journal vol. 40 87–165 Preprint at 10.1093/eurheartj/ehy394 (2019). - PubMed
-
- Neumann, F. J. et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. European Heart Journal vol. 41 407–477 Preprint at 10.1093/eurheartj/ehz425 (2020). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

