Membrane mechanics dictate axonal pearls-on-a-string morphology and function
- PMID: 39623218
- PMCID: PMC11706780
- DOI: 10.1038/s41593-024-01813-1
Membrane mechanics dictate axonal pearls-on-a-string morphology and function
Abstract
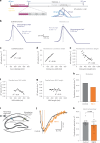
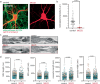
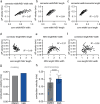
Axons are ultrathin membrane cables that are specialized for the conduction of action potentials. Although their diameter is variable along their length, how their morphology is determined is unclear. Here, we demonstrate that unmyelinated axons of the mouse central nervous system have nonsynaptic, nanoscopic varicosities ~200 nm in diameter repeatedly along their length interspersed with a thin cable ~60 nm in diameter like pearls-on-a-string. In silico modeling suggests that this axon nanopearling can be explained by membrane mechanical properties. Treatments disrupting membrane properties, such as hyper- or hypotonic solutions, cholesterol removal and nonmuscle myosin II inhibition, alter axon nanopearling, confirming the role of membrane mechanics in determining axon morphology. Furthermore, neuronal activity modulates plasma membrane cholesterol concentration, leading to changes in axon nanopearls and causing slowing of action potential conduction velocity. These data reveal that biophysical forces dictate axon morphology and function, and modulation of membrane mechanics likely underlies unmyelinated axonal plasticity.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures










Update of
-
Membrane mechanics dictate axonal morphology and function.bioRxiv [Preprint]. 2023 Jul 21:2023.07.20.549958. doi: 10.1101/2023.07.20.549958. bioRxiv. 2023. Update in: Nat Neurosci. 2025 Jan;28(1):49-61. doi: 10.1038/s41593-024-01813-1. PMID: 37503105 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
- S10 RR026445/RR/NCRR NIH HHS/United States
- 1R01 NS105810-01A1/Foundation for the National Institutes of Health (Foundation for the National Institutes of Health, Inc.)
- MURI FA9550-18-0051/United States Department of Defense | United States Air Force | AFMC | Air Force Office of Scientific Research (AF Office of Scientific Research)
- 1RF1DA055668-01/Foundation for the National Institutes of Health (Foundation for the National Institutes of Health, Inc.)
- 1R35NS132153-01/Foundation for the National Institutes of Health (Foundation for the National Institutes of Health, Inc.)
- S10OD023548/Foundation for the National Institutes of Health (Foundation for the National Institutes of Health, Inc.)
- R01 MH139350/MH/NIMH NIH HHS/United States
- R35 NS132153/NS/NINDS NIH HHS/United States
- R25NS063307/Foundation for the National Institutes of Health (Foundation for the National Institutes of Health, Inc.)
- S10RR026445/Foundation for the National Institutes of Health (Foundation for the National Institutes of Health, Inc.)
- R01 NS105810/NS/NINDS NIH HHS/United States
- R25 NS063307/NS/NINDS NIH HHS/United States
- DP2 NS111133/NS/NINDS NIH HHS/United States
- DGE-2139757/National Science Foundation (NSF)
- RF1 DA055668/DA/NIDA NIH HHS/United States
- 1DP2 NS111133-01/Foundation for the National Institutes of Health (Foundation for the National Institutes of Health, Inc.)
LinkOut - more resources
Full Text Sources
Miscellaneous

