Subcutaneous inoculation of Escherichia coli in broiler chickens causes cellulitis and elicits innate and specific immune responses
- PMID: 39623373
- PMCID: PMC11610265
- DOI: 10.1186/s12917-024-04392-2
Subcutaneous inoculation of Escherichia coli in broiler chickens causes cellulitis and elicits innate and specific immune responses
Abstract
Background: Cellulitis caused by Escherichia coli is a common cause of condemnation of broiler chickens at slaughter worldwide and is associated with economic losses and a possible negative impact on animal welfare. The study objective was to monitor clinical signs and immune responses after subcutaneous E. coli inoculation (1.1-1.8 × 107 CFU), aiming to induce cellulitis. Three groups of broiler chickens (n = 15/group) were inoculated with well-characterized E. coli strains (group A: ECA18 O24:H4/ST117 and group B: ECB11 O153:H9/ST38) or with saline (control) at 22 days-of-age. Clinical signs of disease, body weight and immune parameters were monitored until euthanasia 12-14 days after inoculation followed by post-mortem examination.
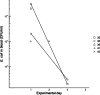
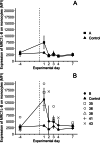
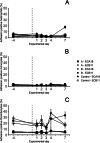
Results: The daily weight gain of the inoculated chickens was significantly lower one day after inoculation compared to the controls. Seven (23%) of the inoculated chickens displayed clinical signs: ruffled feathers, mild weakness, open-beak breathing and/or reluctance to stand, of which two birds were euthanized and one bird died. Five chickens in group B were observed with bacteraemia, which lasted up to three days after inoculation for two chickens. A transient increase in chicken mannose receptor MRC1L-B expression on circulating monocytes was observed one day after inoculation in both E. coli inoculated groups, with a more pronounced increase in group B. On day 7 after inoculation, the in vitro adherence of heterophils, monocytes and thrombocytes to the inoculated strain was increased in group B. Antibody titers to the inoculation strains were increased in some chickens in both groups on days 7 and 14 after inoculation, with the highest titers in group B. Seven (47%) and 13 (87%) of the chickens in group A and B, respectively, were diagnosed with cellulitis at post-mortem examination. In most birds, lesions consisted of plaque-like material embedded in the subcutaneous tissue of the abdominal wall.
Conclusions: Inoculation of E. coli caused cellulitis and prompted a rapid activation/redistribution of circulating monocytes followed by antibody production. The responses were most pronounced in chickens inoculated with E. coli strain ECB11, presumably because of a higher virulence.
Keywords: Post-mortem findings; APEC; Cellulitis; Chicken mannose receptor MRC1L-B; Clinical signs; IgY; Immune response.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The research has been conducted in accordance with the formal approval from the Regional Ethics Committee on Animal Experiments in Uppsala, Sweden (reference number 5.8.18–12116/2020) according to Swedish legislation and directives (SJVFS 2019:9 L 150) based on the European Union legislation directive 2010/63/EU. Chickens were housed at the Swedish Livestock Research Centre of the Swedish University of Agricultural Sciences, approved for experimental animals by the Swedish Board of Agriculture. The study was conducted in compliance with the ARRIVE 2.0 Essential 10 list guidelines. Consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures




References
-
- Majewski, M, Alban, L, Jansson, DS, Lazou, T, Langkabel, N, Antic, D, Kaukonen, E, Wall, H, Pinto, MV, Østergaard, L, Nielsen, OG, Ghidini, S & Sandberg, M: Development of a harmonized and risk-based code system for post-mortem inspection of broilers. Food Control. 2024, 110665.
-
- Nolan LK, Vaillancourt J-P, Barbieri NL, Logue CM, et al. Colibacillosis. In: Swayne DE, Boulianne M, Logue CM, McDougald LR, Nair V, Suarez DL, de Wit S, Grimes T, Johnson D, Kromm M, et al., editors. Diseases of Poultry. 14th ed. Hoboken, NJ: Wiley-Blackwell; 2020. p. 770–830.
-
- Messier S, Quessy S, Robinson Y, Devriese LA, Hommez J, Fairbrother JM. Focal dermatitis and cellulitis in broiler chickens: bacteriological and pathological findings. Avian Dis. 1993;37(3):839–44. - PubMed
-
- Peighambari SM, Julian RJ, Vaillancourt JP, Gyles CL. Escherichia coli cellulitis: experimental infections in broiler chickens. Avian Dis. 1995;39(1):125–34. - PubMed
-
- Randall CJ, Meakins PA, Harris MP, Watt DJ. A new skin disease in broilers? Vet Rec. 1984;114(10):246. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

