NF-κB associated markers of prognosis in early and metastatic triple negative breast cancer
- PMID: 39623404
- PMCID: PMC11613493
- DOI: 10.1186/s13058-024-01925-3
NF-κB associated markers of prognosis in early and metastatic triple negative breast cancer
Abstract
Background: Triple negative breast cancer (TNBC) is the most aggressive subtype of breast cancer. While PD-1 based immunotherapies overall have led to improved treatment outcomes for this disease, a diverse response to frontline chemotherapy and immunotherapy still exist in TNBC, highlighting the need for more robust prognostic markers.
Methods: Tumor-intrinsic immunotranscriptomics, serum cytokine profiling, and tumor burden studies were conducted in two syngeneic mouse models to assess differential effects in both the early-stage and metastatic setting. Bioinformatic analyses of both early and metastatic TNBC patient data were performed to assess if identified NF-κB-associated factors are associated with improved patient clinical outcomes.
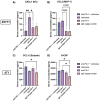
Results: NF-κB signaling driven by lymphotoxin beta expression is associated with tumor regression in TNBC mouse models. Furthermore, lymphotoxin beta expression in patient TNBC cohorts is prognostic of improved survival outcomes.
Conclusions: This study highlights the potential role for NF-κB-associated factors, specifically lymphotoxin beta to be used as prognostic markers in TNBC, which could ultimately provide insight for improved targeted treatment approaches in the clinic.
Keywords: Biomarkers; Immune checkpoint inhibitors; Immunotherapy; NF-κB pathway; Triple negative breast cancer.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was conducted in compliance with guidelines of the Declaration of Helsinki, Belmont report, and U.S. Common rule. Analysis of human patient data was performed utilizing retrospective, deidentified clinical data. All animal protocols were approved by the Brown University Animal Care and Use Committee (#22-09-0002) and were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All animal protocols were reviewed and acknowledged by the Lifespan University Institutional Care and Use Committee (#1987412-1). Consent for publication: Not applicable. Competing interests: JRR is currently an employee at Caris Life Sciences however, her she was an employee of Women and Infants Hospital at the time this research was conducted. All other authors declare no competing interests.
Figures









References
-
- Cortes J, Cescon DW, Rugo HS, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020;396(10265):1817–28. 10.1016/S0140-6736(20)32531-9. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

