Doxorubicin synergizes bortezomib-induced multiple myeloma cell death by inhibiting aggresome formation and augmenting endoplasmic reticulum/Golgi stress and apoptosis
- PMID: 39623468
- PMCID: PMC11613951
- DOI: 10.1186/s12967-024-05920-2
Doxorubicin synergizes bortezomib-induced multiple myeloma cell death by inhibiting aggresome formation and augmenting endoplasmic reticulum/Golgi stress and apoptosis
Abstract
Background: Bortezomib is a standard treatment for multiple myeloma (MM), working by the accumulation of toxic misfolded proteins in cancer cells. However, a significant clinical challenge arises from the development of resistance to bortezomib in MM treatment. Aggresome, a subcellular structure enclosed within Vimentin, forms in response to proteasome inhibitors and sequesters misfolded proteins that are transported by histone deacetylase 6 (HDAC6) and Dynein for degradation via autophagy, thereby reducing bortezomib's cytotoxic effects. Therefore, in this study, we screened several anticancer agents to identify those that could synergize with bortezomib to enhance cell death and block aggresome formation in the MM cell line U266B1.
Methods: To enhance bortezomib's efficacy, we screened a range of anticancer compounds for their potential to promote cell death and inhibit aggresome formation in U266B1 MM cells. We utilized the trypan blue exclusion assay and immunofluorescence for evaluation, and explored the underlying mechanisms through Western blot analysis.
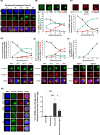
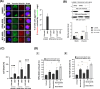
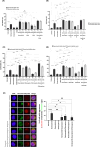
Results: Doxorubicin enhanced bortezomib-induced cytotoxicity while inhibiting aggresome formation. Mechanistic studies revealed that doxorubicin downregulated key aggresome components, including Vimentin, HDAC6, and Dynein, leading to accumulation of misfolded proteins and augmentation of proapoptotic and necroptotic pathways by intensifying endoplasmic reticulum (ER) and Golgi stress responses. Notably, doxorubicin did not enhance cell death triggered by proteasome inhibitors that do not induce aggresome formation. Furthermore, the combination of bortezomib and doxorubicin failed to produce synergy in the killing of MM cell lines that lacked aggresome-forming ability.
Conclusions: Doxorubicin enhances bortezomib-induced cell death in MM by inhibiting aggresome formation and amplifying ER/Golgi stress and apoptosis. This study highlights the potential therapeutic benefits of combining bortezomib with doxorubicin for MM treatment.
Keywords: Aggresome; Bortezomib; Doxorubicin; Golgi stress; Multiple myeloma.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: Chieh-Lin Jerry Teng received honorarium and consulting fees from Novartis, Roche, Pfizer, Takeda, Johnson and Johnson, Amgen, BMS Celgene, Kirin, TTY, and MSD. The other authors have no conflicts of interest.
Figures




References
-
- Gomes CM. Protein misfolding in disease and small molecule therapies. Curr Top Med Chem. 2012;12(22):2460–9. - PubMed
-
- Cheng B, Li Y, Ma L, Wang Z, Petersen RB, Zheng L, et al. Interaction between amyloidogenic proteins and biomembranes in protein misfolding diseases: mechanisms, contributors, and therapy. Biochim Biophys Acta Biomembr. 2018;1860(9):1876–88. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

