Elevated Soluble ACE2 Activity in Children and Adults After SARS-CoV-2 Exposure Irrespective of Laboratory-Confirmed Infection
- PMID: 39624009
- PMCID: PMC11612704
- DOI: 10.1002/jmv.70098
Elevated Soluble ACE2 Activity in Children and Adults After SARS-CoV-2 Exposure Irrespective of Laboratory-Confirmed Infection
Abstract
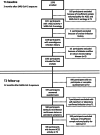
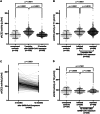
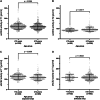
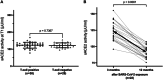
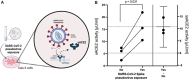
The pivotal role of the cell entry receptor ACE2 for SARS-CoV-2 infection is well-established. When ACE2 is shed from cell surface into plasma as soluble ACE2 (sACE2), it can effectively neutralize SARS-CoV-2. This longitudinal prospective cohort study analyzed sACE2 activity in 1192 participants, aged 4 months to 81 years, 3 and 12 months after SARS-CoV-2 household exposure. Following SARS-CoV-2 exposure, participants exhibited significantly elevated sACE2 activity, irrespective of confirmed infection, with the highest levels observed in exposed children. Longitudinal analysis revealed a decline in sACE2 levels over time, reaching levels comparable to age- and sex-matched pre-pandemic controls. An increase in sACE2 activity was also confirmed in vitro in Calu-3 (human lung) cells within hours of SARS-CoV-2 exposure, providing a direct link between SARS-CoV-2 exposure and elevated sACE2. This study, therefore, challenges the dichotomy of categorizing SARS-CoV-2 exposed participants as infected or not infected solely on currently established diagnostic assays. It demonstrates lasting host responses independent of B- and T-cell memory and may help to keep SARS-CoV-2 infections in balance and contribute to successful virus clearance in children and adults lacking humoral and cellular immune responses following SARS-CoV-2 exposure. Trial Registration: German Registry for Clinical Studies; Identifier: D 00021521.
Keywords: ACE2; SARS‐CoV‐2; children; exposure; persistence.
© 2024 The Author(s). Journal of Medical Virology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

