Efficacy of a Tumor Necrosis Factor Inhibitor in Chronic Low-Back Pain With Modic Type 1 Changes: A Randomized Controlled Trial
- PMID: 39624017
- PMCID: PMC12039465
- DOI: 10.1002/art.43073
Efficacy of a Tumor Necrosis Factor Inhibitor in Chronic Low-Back Pain With Modic Type 1 Changes: A Randomized Controlled Trial
Abstract
Objective: The efficacy of tumor necrosis factor inhibitors for treating chronic low-back pain with Modic changes is uncertain. This study investigated the superiority of infliximab over placebo in patients with Modic type 1 changes.
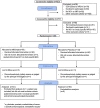
Methods: In this multicenter, randomized, triple-blind, placebo-controlled trial, patients aged 18 to 65 years with moderate to severe chronic low-back pain and Modic type 1 changes were enrolled from five Norwegian public hospitals between January 2019 and October 2022. Participants were randomly assigned to four intravenous infusions of 5 mg/kg infliximab or placebo. The primary outcome was difference in change in the Oswestry Disability Index (ODI) score from baseline to five months. Secondary outcomes included changes in low-back pain intensity, disability, and health-related quality of life. A linear mixed model was used for efficacy analyses.
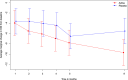
Results: A total of 128 patients (mean age 43 years, 65.6% women) participated (64 in each group). All patients who received at least one dose of the allocated infusion were included in the primary analyses. The average ODI score (±SD) change was -7.0 (±9.7) in the group who received infliximab and -6.4 (±10.4) in the group who received placebo. The difference in the ODI score change between the two groups was 1.3 ODI points (95% confidence interval -2.1 to 4.6, P = 0.45). Analyses showed no effect of infliximab compared to placebo on secondary outcomes. Adverse event rates were similar between groups.
Conclusion: Infliximab did not demonstrate superiority over placebo in reducing pain-related disability in patients with moderate to severe chronic low-back pain with Modic type 1 changes at five months.
© 2024 The Author(s). Arthritis & Rheumatology published by Wiley Periodicals LLC on behalf of American College of Rheumatology.
Figures
References
-
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990‐2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392(10159):1789–1858. - PMC - PubMed
-
- Hartvigsen J, Hancock MJ, Kongsted A, et al. What low back pain is and why we need to pay attention. Lancet 2018;391(10137):2356–2367. - PubMed
-
- Knezevic NN, Candido KD, Vlaeyen JWS, et al. Low back pain. Lancet 2021;398(10294):78–92. - PubMed
-
- Foster NE, Anema JR, Cherkin D, et al. Prevention and treatment of low back pain: evidence, challenges, and promising directions. Lancet 2018;391(10137):2368–2383. - PubMed
-
- Maher C, Underwood M, Buchbinder R. Non‐specific low back pain. Lancet 2017;389(10070):736–747. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

