Global differences and risk factors influencing drug hypersensitivity quality of life: A multicenter, multiethnic study of drug allergy across 3 continents
- PMID: 39624181
- PMCID: PMC11609553
- DOI: 10.1016/j.jacig.2024.100354
Global differences and risk factors influencing drug hypersensitivity quality of life: A multicenter, multiethnic study of drug allergy across 3 continents
Abstract
Background: Penicillin allergy labels are associated with many adverse outcomes. Fear and restriction of future medication use also have an impact on health-related quality of life (HR-QoL). However, the impact of a drug allergy on HR-QoL and its associated factors remains unknown.
Objective: We sought to investigate the impact of penicillin allergy labels and compare the factors associated with HR-QoL impairment among patients in an international multicenter, multiethnic cohort.
Methods: HR-QoL was measured using the 6-item Drug Hypersensitivity Quality of Life Questionnaire (DrHy-Q) and compared among patients labeled with penicillin allergy, before their allergy evaluation, from 8 adult allergy/immunology clinics across Asia, Australia, and North America.
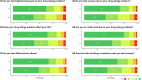
Results: We recruited 643 patients labeled with penicillin allergy (median age, 56 years [interquartile range, 39-67]; male:female ratio, 1:2.2), with 273 (42.5%), 186 (28.9%), and 184 (28.6%) from Asia, North America, and Australia, respectively. The median DrHy-Q score was 8.3 (interquartile range, 0.0-29.2). All patients underwent penicillin allergy evaluation, and 96% (617 of 643) were delabeled following negative provocation test results. Female patients (8.3 vs 4.2; P = .003), those with other concomitant antimicrobial allergy labels (20.8 vs 4.2; P = .004), and patients from Asia (33.3 vs 4.2 [North America] vs 0 [Australia]; P < .001) had significantly higher DrHy-Q scores, reflecting a reduced HR-QoL. Ethnicity as well as other allergy variables were not significant in the multivariate analysis.
Conclusions: Regional differences, ethnicity, and other risk factors influence HR-QoL impairment among patients labeled with penicillin allergy. Future studies are needed to understand the contributions of regional sociodemographic factors and identify interventions to improve HR-QoL.
Keywords: Drug allergy; delabeling; health-related quality of life (HR-QoL); penicillin; quality of life.
© 2024 The Authors.
Conflict of interest statement
A.M.C. received support from the Montreal General Hospital Foundation and the Research Institute of the McGill University Health Centre and was awarded the University of Melbourne Research Scholarship, the Anna Maria Solinas Laroche Career Award in Immunology, and the Anita Garbarino Girard/Anna Maria Solinas/Dr Phil Gold Award of Distinction. J.A.T. was supported by the Austin Medical Research Foundation and by a National Health and Medical Research Council postgraduate scholarship (no. GNT 1139902). Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
Figures




References
-
- Wong J.C.Y., Kan A.K.C., Chik T.S.H., Chu M.Y., Li T.C.M., Mak H.W.F., et al. Prospective, multicenter, head-to-head comparison between allergists versus nonallergists in low-risk penicillin allergy delabeling: effectiveness, safety, and quality of life (HK-DADI2) J Allergy Clin Immunol Pract. 2024;12:1801–1808.e2. - PubMed
-
- Li TS, Hui HKS, Kan AKC, Yeung MHY, Wong JCY, Chiang V, et al. Prospective Assessment of Penicillin Allergy (PAPA): evaluating the performance of penicillin allergy testing and post-delabelling outcomes among Hong Kong Chinese [published online ahead of print April 17, 2023]. Asian Pac J Allergy Immunol. 10.12932/AP-270922-1469. - DOI - PubMed
LinkOut - more resources
Full Text Sources

