Pulmonary fibrosis in patients with autoimmune pulmonary alveolar proteinosis: a retrospective nationwide cohort study
- PMID: 39624377
- PMCID: PMC11610044
- DOI: 10.1183/23120541.00314-2024
Pulmonary fibrosis in patients with autoimmune pulmonary alveolar proteinosis: a retrospective nationwide cohort study
Abstract
Background: Autoimmune pulmonary alveolar proteinosis (aPAP) is a rare disease that may progress towards pulmonary fibrosis. Data about fibrosis prevalence and risk factors are lacking.
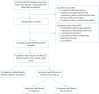
Methods: In this retrospective multicentre nationwide cohort, we included patients newly diagnosed with aPAP between 2008 and 2018 in France and Belgium. Data were collected from medical records using a standardised questionnaire.
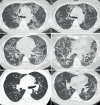
Results: 61 patients were included in the final analysis. We identified 5 patients (8%) with fibrosis on initial computed tomography (CT) and 16 patients (26%) with fibrosis on final CT after a median time of 3.6 years. Dust exposure was associated with pulmonary fibrosis occurrence (OR 4.3; p=0.038). aPAP patients treated with whole-lung lavage, rituximab or granulocyte-monocyte colony-stimulating factor therapy did not have more fibrotic evolution than patients who did not receive these treatments (n=25 out of 45, 57% versus n=10 out of 16, 62%; p=0.69). All-cause mortality was significantly higher in fibrotic than in nonfibrotic cases (n=4 out of 16, 25% versus n=2 out of 45, 4.4%; p=0.036, respectively).
Conclusion: In our population, a quarter of aPAP patients progressed towards pulmonary fibrosis. Dust exposure seems to be an important factor associated with this complication. More studies are needed to analyse precisely the impact of dust exposure impact, especially silica, in patients with aPAP.
Copyright ©The authors 2024.
Conflict of interest statement
Conflict of interest: C. Chenivesse declares having received grants from AstraZeneca, GlaxoSmithKline, Novartis and Santelys, personal fees from ALK-Abello, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline and Sanofi, and congress support from AstraZeneca, Boehringer Ingelheim, Chiesi, Novartis and Sanofi. M. Lederlin has received fees, funding or reimbursement for national and international conferences, boards, expert or opinion groups and research projects over the past 5 years from AstraZeneca, Boehringer, Fresenius-Kabi and Siemens Healthcare. S. Jouneau has received fees, funding or reimbursement for national and international conferences, boards, expert or opinion groups, and research projects over the past 5 years from Actelion, AIRB, AstraZeneca, Bellorophon Therapeutics, Biogen, BMS, Boehringer, Chiesi, Fibrogen, Gilead, GSK, LVL, Mundipharma, Novartis, Olam Pharm, Pfizer, Pliant Therapeutics, Roche and Savara-Serendex. The other authors have nothing to disclose.
Figures

References
LinkOut - more resources
Full Text Sources
