Biomarkers troponin and procalcitonin in addition to CRB-65 enhance risk stratification in patients with community-acquired pneumonia
- PMID: 39624382
- PMCID: PMC11609962
- DOI: 10.1183/23120541.00420-2024
Biomarkers troponin and procalcitonin in addition to CRB-65 enhance risk stratification in patients with community-acquired pneumonia
Abstract
Background: Community-acquired pneumonia (CAP) remains a leading cause of infectious disease mortality globally, necessitating intensive care unit (ICU) admission for ∼10% of hospitalised patients. Accurate prediction of disease severity facilitates timely therapeutic interventions.
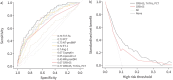
Methods: Our study aimed to enhance the predictive capacity of the clinical CRB-65 score by evaluating eight candidate biomarkers: troponin T high-sensitive (TnT-hs), procalcitonin (PCT), N-terminal pro-brain natriuretic peptide, angiopoietin-2, copeptin, endothelin-1, lipocalin-2 and mid-regional pro-adrenomedullin. We utilised a machine-learning approach on 800 samples from the German CAPNETZ network (competence network for CAP) to refine risk prediction models combining these biomarkers with the CRB-65 score regarding our defined end-point: death or ICU admission during the current CAP episode within 28 days after study inclusion.
Results: Elevated levels of biomarkers were associated with the end-point. TnT-hs exhibited the highest predictive performance among individual features (area under the receiver operating characteristic curve, AUC=0.74), followed closely by PCT (AUC=0.73). Combining biomarkers with the CRB-65 score significantly improved prediction accuracy. The combined model of CRB-65, TnT-hs and PCT demonstrated the best balance between high predictive value and parsimony, with an AUC of 0.77 (95% CI: 0.72-0.82), while CRB-65 alone achieved an AUC of 0.67 (95% CI: 0.64-0.73).
Conclusion: Our findings suggest that augmenting the CRB-65 score with TnT-hs and PCT enhances the prediction of death or ICU admission in hospitalised CAP patients. Validation of this improved risk score in additional CAP cohorts and prospective clinical studies is warranted to assess its broad clinical utility.
Copyright ©The authors 2024.
Conflict of interest statement
Conflict of interest: M. Scholz received funding from Pfizer Inc. for epidemiological modelling of pneumococcal serotypes and vaccinations, and has an ongoing cooperation with Owkin for a project not related to this research. M. Witzenrath received funding from Aptarion, Biotest and Pantherna for research outside the current study. The remaining authors have nothing to disclose.
Figures

References
LinkOut - more resources
Full Text Sources
Miscellaneous
