Acute exacerbations in patients with progressive pulmonary fibrosis
- PMID: 39624387
- PMCID: PMC11610068
- DOI: 10.1183/23120541.00403-2024
Acute exacerbations in patients with progressive pulmonary fibrosis
Abstract
Background: Acute exacerbations of fibrosing interstitial lung diseases (ILDs) are associated with high mortality. We used prospective data from the INBUILD trial to investigate risk factors for acute exacerbations and the impact of these events in patients with progressive pulmonary fibrosis.
Methods: Patients with progressive fibrosing ILDs other than idiopathic pulmonary fibrosis (IPF) were randomised to receive nintedanib or placebo. Associations between baseline characteristics and time to first acute exacerbation were assessed using pooled data from both treatment groups using Cox proportional hazard models, firstly univariable models and then a multivariable model using forward stepwise selection. The risk of death was estimated based on the Kaplan-Meier method.
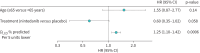
Results: Over a median follow-up of approximately 19 months, acute exacerbations were reported in 58 (8.7%) of 663 patients. In the risk factor analysis, the final model included diffusing capacity of the lung for carbon monoxide (D LCO) % predicted, treatment and age. Lower D LCO % predicted was associated with an increased risk of acute exacerbation with a hazard ratio (HR) of 1.56 (95% CI 1.21-2.02) per 10 units lower (p<0.001). Age ≥65 years was associated with a numerically increased risk (HR 1.55, 95% CI 0.87-2.77; p=0.14). Treatment with nintedanib conferred a numerically reduced risk versus placebo (HR 0.60, 95% CI 0.35-1.02; p=0.06). The estimated risks of death ≤30 days and ≤90 days after an acute exacerbation were 19.0% (95% CI 8.9-29.2) and 32.0% (95% CI 19.7-44.2).
Conclusions: Acute exacerbations of progressive pulmonary fibrosis may have similar risk factors and prognostic impact as acute exacerbations of IPF.
Copyright ©The authors 2024.
Conflict of interest statement
Conflict of interest: M. Kreuter reports grants, consulting fees and fees for speaking from Boehringer Ingelheim and Roche; and holds leadership or fiduciary roles with the Deutsche Gesellschaft für Pneumologie, European Respiratory Society and German Respiratory Society. E.A. Belloli reports fees from Boehringer Ingelheim for participation in an advisory board meeting. E. Bendstrup reports an unrestricted grant from Boehringer Ingelheim; fees for speaking from Boehringer Ingelheim, Chiesi, Daiichi Sankyo, GlaxoSmithKline, AstraZeneca and Roche; support for travel from Boehringer Ingelheim and Roche; and has participated on Data Safety Monitoring Boards or advisory boards for AbbVie, Veracyte and Boehringer Ingelheim. S. Cerri reports fees for speaking from Boehringer Ingelheim. K.R. Flaherty reports grants paid to his institution from Boehringer Ingelheim; royalties from UpToDate; consulting fees from Arrowhead, AstraZeneca, Bellerophon, CSL Behring, Daewoong, DevPro, Dispersol, FibroGen, Horizon, Immunet, Insilico, Lupin, NeRRe, Pliant, Polarean, Pure Health, PureTech, Respivant, Roche/Genentech, Shionogi, Sun Pharmaceuticals, Trevi, United Therapeutics and Vicore; he is a Steering Committee Chair for the Pulmonary Fibrosis Foundation and was a member of the INBUILD trial Steering Committee. S. Shapera reports grants to support fellowship training from the Canadian Pulmonary Fibrosis Foundation; has participated on advisory boards for AstraZeneca and Hoffmann-La Roche; and has received fees for speaking from Boehringer Ingelheim and AstraZeneca. J.W. Song was supported by grants from the Basic Science Research Program and the Bio & Medical Technology Development Program of the National Research Foundation of Korea funded by the Ministry of Science & ICT and is supported by a National Institute of Health research project and by the Korea Environment Industry & Technology Institute through Core Technology Development Project for Environmental Diseases Prevention and Management Program funded by the Korea Ministry of Environment; he reports fees for consulting or lectures from Boehringer Ingelheim, BMS, Taiho and Daewoong. H. Mueller and K.B. Rohr are employees of Boehringer Ingelheim. Y. Kondoh reports consulting fees from Asahi Kasei, Boehringer Ingelheim, Chugai, Healios, Janssen, Shionogi and Taiho; and fees for lectures from Asahi Kasei, Boehringer Ingelheim, Bristol Myers Squibb, Eisai, Janssen, KYORIN, Mitsubishi Tanabe, Nippon Shinyaku, Novartis, Shionogi and Teijin.
Figures


References
LinkOut - more resources
Full Text Sources
