Apomorphine for prolonged disorders of consciousness: a multimodal open-label study
- PMID: 39624459
- PMCID: PMC11609466
- DOI: 10.1016/j.eclinm.2024.102925
Apomorphine for prolonged disorders of consciousness: a multimodal open-label study
Abstract
Background: Apomorphine is a dopaminergic candidate therapy to improve recovery in patients with prolonged disorders of consciousness (PDoC). Behavioural improvements were previously described in non-controlled case series, but its efficacy and neural mechanisms remain largely unknown. This open-label controlled study using multimodal outcome measures investigates the action of apomorphine in severely brain-injured patients.
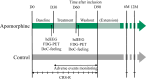
Methods: Thirteen PDoC patients received 30-day subcutaneous apomorphine treatment (n = 6) or standard care (control group, n = 7) in a neurological rehabilitation centre between February 2018 and January 2021. The apomorphine group was monitored 30 days before treatment initiation, during treatment and one year after treatment. Primary outcome measure was defined as changes in behavioural diagnosis using the Coma Recovery Scale-Revised (CRS-R). CRS-R index, recovery of new conscious behaviours, DoC-feeling scores, high-density electroencephalography, and fluorodeoxyglucose positron emission tomography were employed as secondary outcome measures. The control group was monitored with repeated CRS-R only. Registration: EudraCT 2018-003144-23; Clinicaltrials.govNCT03623828.
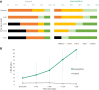
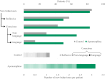
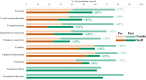
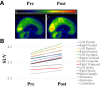
Findings: Groups (apomorphine vs. control: odds ratio 8.9, 95% CI 3.3-17.8) and study phase (treatment vs. baseline, apomorphine group only: odds ratio 3.9, 95% CI 1.5-10.1) significantly influenced positive changes in behavioural diagnosis. At one-year post-injury, 4/6 patients in the apomorphine group and 1/7 patients in the control group had improved their diagnosis. Similarly, CRS-R index was significantly influenced by study phase (treatment vs. baseline). All items on the DoC-feeling score were rated higher after treatment than before by both family and medical staff. Patients in the apomorphine group recovered more conscious behaviours than control patients. Alpha-band whole-brain connectivity and participation coefficient, as well as alpha-band parieto-temporal connectivity and frontal participation coefficient were higher after treatment than at baseline. Whole-brain metabolism increased by a relative mean of 13.8% after treatment compared to baseline, with a significant effect of timing (pre-vs. post-treatment scans) on regional SUV.
Interpretation: Long-lasting consciousness improvements were observed in patients treated with apomorphine, compared to controls and compared to baseline. Changes in brain connectivity and metabolism were observed after treatment, providing insights into possible neurophysiological mechanisms and target areas. This open-label study confirmed the feasibility and safety of apomorphine treatment, which may represent a key therapeutic option for PDoC.
Funding: University and University Hospital of Liege, Belgian National Funds for Scientific Research, Fund Generet of the King Baudouin Foundation, AstraZeneca Foundation, Leon Fredericq Foundation and NeuroHealing Pharmaceuticals Inc.
Keywords: Apomorphine; Coma; Disorders of consciousness; High-density electroencephalography; Minimally conscious state; Neuroimaging; Positron emission tomography; Severe brain injury; Treatment; Unresponsive wakefulness syndrome; Vegetative state.
© 2024 The Authors.
Conflict of interest statement
Dr Sanz and Dr Gosseries report grants from the Belgian National Funds for Scientific Research (FRS-FNRS), grants from Fund Generet (King Baudouin Foundation), grants from Foundation Leon Fredericq, grants from European Union Horizon 2020 FP, grants from AstraZeneca Foundation, grants from DOCMA project (EU-H2020-MSCA-RISE-778234), and grants from Neurohealing Pharmaceuticals, during the conduct of the study. Dr. Huerta-Gutierrez reports funding from the German Academic Exchange Service (DAAD). Dr Zafonte was partially supported by NIDILRR, NIH and USARMC and receives funding from the Football Players Health Study at Harvard University, which is funded by the NFL Players Association. Dr Zafonte received royalties from Springer/Demos publishing for serving as co-editor of the text Brain Injury Medicine. Dr Zafonte serves on the Scientific Advisory Board of Myomo and Kisbee. In the past, he has advised Nanodx. He also evaluates patients in the MGH Brain and Body-TRUST Program which is funded by the NFL Players Association.
Figures



References
-
- Giacino J.T., Fins J.J., Laureys S., Schiff N.D. Disorders of consciousness after acquired brain injury: the state of the science. Nat Rev Neurol. 2014;10:99–114. - PubMed
-
- Giacino J.T., Ashwal S., Childs N., et al. The minimally conscious state: definition and diagnostic criteria. Neurology. 2002;58:349–353. - PubMed
-
- Thibaut A., Bodien Y.G., Laureys S., Giacino J.T. Minimally conscious state “plus”: diagnostic criteria and relation to functional recovery. J Neurol. 2020;267:1245–1254. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

