Outcomes of Concomitant Atrial Fibrillation Ablation and Left Atrial Appendage Closure: A Retrospective Single-Center Experience
- PMID: 39624573
- PMCID: PMC11609395
- DOI: 10.1016/j.jacadv.2024.101377
Outcomes of Concomitant Atrial Fibrillation Ablation and Left Atrial Appendage Closure: A Retrospective Single-Center Experience
Abstract
Background: Catheter ablation is an effective therapy in the management of atrial fibrillation (AF). Left atrial appendage closure (LAAC) is an alternative to anticoagulation for stroke prevention in patients with bleeding risks.
Objectives: The purpose of this study was to assess the safety and efficacy of combining AF ablation and LAAC in a single procedure.
Methods: This retrospective observational study included consecutive patients who underwent concomitant AF ablation and LAAC between June 2015 and August 2021. We assessed the safety and efficacy of the combined procedure with respect to procedural success, periprocedural complications, thromboembolism, bleeding, and arrhythmia recurrence.
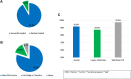
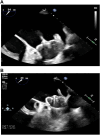
Results: A total of 178 patients (mean age 72.1 ± 8.7 years, 60.7% male) were identified. The mean CHA2DS2VASc and HAS-BLED scores were 4.0 ± 1.4 and 2.8 ± 1.1, respectively. Pulmonary vein isolation was achieved in all patients. LAAC was aborted in 15 cases (success rate of 91.6%). The periprocedural complications rate was 6.2%. The median follow-up duration was 412 days IQR: 213 to 781 days. There were 2 strokes and 3 transient ischemic attacks, equating to an annual risk of 1.7% at 1 year and 4.6% at 2 years. Complete seal was achieved in 97.5% intraprocedurally and in 73.7% on initial follow-up, with no major leaks identified. There were 2 cases (1.3%) of device-related thrombus that resolved with anticoagulation. Thirty-four bleeding events occurred in 28 patients (17.4%). Anticoagulation was discontinued in 93.8% of patients.
Conclusions: Concomitant AF ablation and LAAC could be considered in appropriate patients in centers of clinical expertise.
Keywords: WATCHMAN; atrial fibrillation; catheter ablation; device occlusion; left atrial appendage closure.
© 2024 The Authors.
Conflict of interest statement
Dr Wazni has served as a consultant for Biosense Webster and Boston Scientific. Dr Callahan has served as a consultant for Biotronics and Philips. Dr Santangeli has received speaking honorarium from Boston Scientific. Dr Rickard has received research grant from 10.13039/100008497Boston Scientific, 10.13039/100004374Medtronic, Ireland, and 10.13039/100000046Abbott. Dr Taigen has served as a consultant for Medtronic and Biosense Webster. Dr Jaber has served as a consultant for Boston Scientific and Pfizer. Dr Saliba has served as an advisory board member for Boston Scientific. Dr Kanj has received speaking honorarium from Boston Scientific; and has served as a consultant for Boston Scientific. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures

References
-
- Packer D.L., Mark D.B., Robb R.A., et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321(13):1261. doi: 10.1001/jama.2019.0693. - DOI - PMC - PubMed
-
- Joza J., Samuel M., Jackevicius C.A., et al. Long-term risk of stroke and bleeding post-atrial fibrillation ablation. J Cardiovasc Electrophysiol. 2018;29:1355–1362. - PubMed
LinkOut - more resources
Full Text Sources

