Valproic Acid Inhibits Endoplasmic Reticulum Stress and Reduces Ferroptosis After Traumatic Brain Injury
- PMID: 39624711
- PMCID: PMC11610021
- DOI: 10.1177/15593258241303646
Valproic Acid Inhibits Endoplasmic Reticulum Stress and Reduces Ferroptosis After Traumatic Brain Injury
Abstract
Backgound: Traumatic brain injury (TBI) is a severe neurological disorders, which invloving complicated molecular mechanisms, such as endoplasmic reticulum (ER) stress and ferroptosis. , However, the mechanism underlying TBI remains unclear.
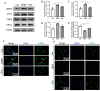
Objectives: The Objective was to determine the effect of VPA on ER stress and ferroptosis, and affirm the relationship between ER stress and ferroptosis. Methods: The expression levels of GRP78, ATF6, CHOP and GPX4 in brain tissues were detected via western blot, histological staining, and immunofluorescence. The effect of VPA on ER stress and ferroptosis on OS cellswas evaluated in vitro and in vivo.
Results: In our study, we found that VPA suppressed ER stress after TBI by inhibiting the GRP78-ATF6-CHOP signaling pathway, which ameliorated ferroptosis by reversing the reduction of the ferroptosis protein GPX4. Furthermore, tissue defects, bleeding, and iron accumulation also reduced. Moreover, 4-phenylbutyric acid was used to further confirm our assumption.
Conclusion: VPA plays a neuroprotective role by inhibiting ER stress levels and subsequently inhibiting ferroptosis.
Keywords: endoplasmic reticulum; ferroptosis; traumatic brain injury; valproic acid.
© The Author(s) 2024.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures







Similar articles
-
rmMANF prevents sepsis-associated lung injury via inhibiting endoplasmic reticulum stress-induced ferroptosis in mice.Int Immunopharmacol. 2023 Jan;114:109608. doi: 10.1016/j.intimp.2022.109608. Epub 2022 Dec 20. Int Immunopharmacol. 2023. PMID: 36700778
-
Valproate Attenuates Endoplasmic Reticulum Stress-Induced Apoptosis in SH-SY5Y Cells via the AKT/GSK3β Signaling Pathway.Int J Mol Sci. 2017 Feb 8;18(2):315. doi: 10.3390/ijms18020315. Int J Mol Sci. 2017. PMID: 28208696 Free PMC article.
-
Didang Tang alleviates neuronal ferroptosis after intracerebral hemorrhage by modulating the PERK/eIF2α/ATF4/CHOP/GPX4 signaling pathway.Front Pharmacol. 2024 Oct 24;15:1472813. doi: 10.3389/fphar.2024.1472813. eCollection 2024. Front Pharmacol. 2024. PMID: 39525631 Free PMC article.
-
Salubrinal offers neuroprotection through suppressing endoplasmic reticulum stress, autophagy and apoptosis in a mouse traumatic brain injury model.Neurobiol Learn Mem. 2019 May;161:12-25. doi: 10.1016/j.nlm.2019.03.002. Epub 2019 Mar 6. Neurobiol Learn Mem. 2019. PMID: 30851432
-
Crosstalk between Endoplasmic Reticulum Stress and Ferroptosis in Liver Diseases.Front Biosci (Landmark Ed). 2024 Jun 20;29(6):221. doi: 10.31083/j.fbl2906221. Front Biosci (Landmark Ed). 2024. PMID: 38940044 Review.
Cited by
-
Indole-3-acetic acid and chenodeoxycholic acid attenuate TLR4/NF-κB signaling and endoplasmic reticulum stress in valproic acid-induced neurotoxicity.Front Pharmacol. 2025 Mar 24;16:1570125. doi: 10.3389/fphar.2025.1570125. eCollection 2025. Front Pharmacol. 2025. PMID: 40196372 Free PMC article.
References
-
- Katz PS, Sulzer JK, Impastato RA, Teng SX, Rogers EK, Molina PE. Endocannabinoid degradation inhibition improves neurobehavioral function, blood-brain barrier integrity, and neuroinflammation following mild traumatic brain injury. J Neurotrauma. 2015;32(5):297-306. doi:10.1089/neu.2014.3508 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

