An acute microglial metabolic response controls metabolism and improves memory
- PMID: 39625057
- PMCID: PMC11614388
- DOI: 10.7554/eLife.87120
An acute microglial metabolic response controls metabolism and improves memory
Abstract
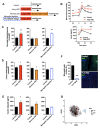
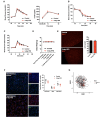
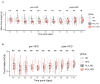
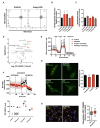
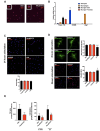
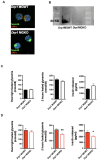
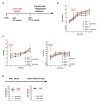
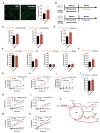
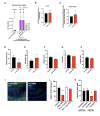
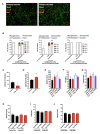
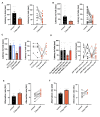
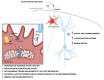
Chronic high-fat feeding triggers metabolic dysfunction including obesity, insulin resistance, and diabetes. How high-fat intake first triggers these pathophysiological states remains unknown. Here, we identify an acute microglial metabolic response that rapidly translates intake of high-fat diet (HFD) to a surprisingly beneficial effect on metabolism and spatial/learning memory. High-fat intake rapidly increases palmitate levels in cerebrospinal fluid and triggers a wave of microglial metabolic activation characterized by mitochondrial membrane activation and fission as well as metabolic skewing toward aerobic glycolysis. These effects are detectable throughout the brain and can be detected within as little as 12 hr of HFD exposure. In vivo, microglial ablation and conditional DRP1 deletion show that the microglial metabolic response is necessary for the acute effects of HFD. 13C-tracing experiments reveal that in addition to processing via β-oxidation, microglia shunt a substantial fraction of palmitate toward anaplerosis and re-release of bioenergetic carbons into the extracellular milieu in the form of lactate, glutamate, succinate, and intriguingly, the neuroprotective metabolite itaconate. Together, these data identify microglia as a critical nutrient regulatory node in the brain, metabolizing away harmful fatty acids and liberating the same carbons as alternate bioenergetic and protective substrates for surrounding cells. The data identify a surprisingly beneficial effect of short-term HFD on learning and memory.
Keywords: cell biology; diabetes; inflammation; memory; metabolism; microglia; mitochondria; mouse; neuroscience.
© 2023, Drougard et al.
Conflict of interest statement
AD, EM, VW, RS, IP, NV, SA, LF, JS, KG, JV, SP, AB, ED, FG, SS, MH, MP, RJ, JP No competing interests declared
Figures


Update of
-
An acute microglial metabolic response controls metabolism and improves memory.bioRxiv [Preprint]. 2024 Apr 25:2023.04.03.535373. doi: 10.1101/2023.04.03.535373. bioRxiv. 2024. Update in: Elife. 2024 Dec 03;12:RP87120. doi: 10.7554/eLife.87120. PMID: 37066282 Free PMC article. Updated. Preprint.
References
-
- Benani A, Hryhorczuk C, Gouazé A, Fioramonti X, Brenachot X, Guissard C, Krezymon A, Duparc T, Colom A, Nédélec E, Rigault C, Lemoine A, Gascuel J, Gerardy-Schahn R, Valet P, Knauf C, Lorsignol A, Pénicaud L. Food intake adaptation to dietary fat involves PSA-dependent rewiring of the arcuate melanocortin system in mice. The Journal of Neuroscience. 2012;32:11970–11979. doi: 10.1523/JNEUROSCI.0624-12.2012. - DOI - PMC - PubMed
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

