The potassium channel subunit KV1.8 (Kcna10) is essential for the distinctive outwardly rectifying conductances of type I and II vestibular hair cells
- PMID: 39625061
- PMCID: PMC11614384
- DOI: 10.7554/eLife.94342
The potassium channel subunit KV1.8 (Kcna10) is essential for the distinctive outwardly rectifying conductances of type I and II vestibular hair cells
Abstract
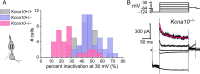
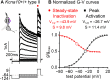
In amniotes, head motions and tilt are detected by two types of vestibular hair cells (HCs) with strikingly different morphology and physiology. Mature type I HCs express a large and very unusual potassium conductance, gK,L, which activates negative to resting potential, confers very negative resting potentials and low input resistances, and enhances an unusual non-quantal transmission from type I cells onto their calyceal afferent terminals. Following clues pointing to KV1.8 (Kcna10) in the Shaker K channel family as a candidate gK,L subunit, we compared whole-cell voltage-dependent currents from utricular HCs of KV1.8-null mice and littermate controls. We found that KV1.8 is necessary not just for gK,L but also for fast-inactivating and delayed rectifier currents in type II HCs, which activate positive to resting potential. The distinct properties of the three KV1.8-dependent conductances may reflect different mixing with other KV subunits that are reported to be differentially expressed in type I and II HCs. In KV1.8-null HCs of both types, residual outwardly rectifying conductances include KV7 (Knq) channels. Current clamp records show that in both HC types, KV1.8-dependent conductances increase the speed and damping of voltage responses. Features that speed up vestibular receptor potentials and non-quantal afferent transmission may have helped stabilize locomotion as tetrapods moved from water to land.
Keywords: hair cell; inner ear; mouse; neuroscience; potassium channel; utricle; vestibular; voltage gated.
© 2024, Martin et al.
Conflict of interest statement
HM, AL, RE No competing interests declared
Figures










Update of
-
The potassium channel subunit KV1.8 (Kcna10) is essential for the distinctive outwardly rectifying conductances of type I and II vestibular hair cells.bioRxiv [Preprint]. 2024 Aug 18:2023.11.21.563853. doi: 10.1101/2023.11.21.563853. bioRxiv. 2024. Update in: Elife. 2024 Dec 03;13:RP94342. doi: 10.7554/eLife.94342. PMID: 38045305 Free PMC article. Updated. Preprint.
References
-
- Alexander SPH, Mathie A, Peters JA, Veale EL, Striessnig J, Kelly E, Armstrong JF, Faccenda E, Harding SD, Pawson AJ, Sharman JL, Southan C, Davies JA, CGTP Collaborators The concise guide to pharmacology 2019/20: ion channels. British Journal of Pharmacology. 2019;176 Suppl 1:S142–S228. doi: 10.1111/bph.14749. - DOI - PMC - PubMed
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

