Plasma p-tau immunoassays in clinical research for Alzheimer's disease
- PMID: 39625101
- PMCID: PMC11977406
- DOI: 10.1002/alz.14397
Plasma p-tau immunoassays in clinical research for Alzheimer's disease
Abstract
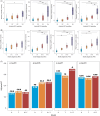
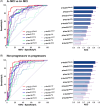
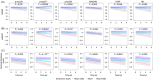
The revised biomarker framework for diagnosis and staging of Alzheimer's disease (AD) relies on amyloid beta (Aβ) and tau pathologies as core markers, and markers for adjacent pathophysiology, such as neurodegeneration and inflammation. Many of the core fluid biomarkers are phosphorylated tau (p-tau) fragments, with p-tau217 showing a prominent association with Aβ and tau. While positron emission tomography (PET) imaging is well established, plasma p-tau assays are newer and likely to reduce the use of expensive, and less accessible cerebrospinal fluid and PET imaging tests, thereby promoting wider access to AD screening. There is a need for greater understanding of how the various plasma p-tau species reflect different pathological processes of AD and how different immunoassays perform. This review surveys the available immunoassays and highlights their strengths and limitations in different contexts of use. Assays need to be standardized to maximize their impact on AD clinical research, and patient diagnosis and management. HIGHLIGHTS: Different plasma phosphorylated tau (p-tau) species reflect different pathological processes of Alzheimer's disease (AD), with p-tau231 showing the greatest association with the earliest increases in brain amyloid beta (Aβ) accumulation, while p-tau217 shows greater association with both brain Aβ and early tau pathology, and other p-tau and tau fragment species show greater association with later stages of brain tau pathology. Plasma p-tau217 has proven to be an excellent biomarker for AD pathology due to its close association with both brain Aβ and tau pathology, as well as its large dynamic range. Many different assays with varying performance exist for the same p-tau species, with mass spectrometry assays performing uniformly well, and several immunoassays achieving comparable performance. "Round robin" head-to-head studies have been performed to compare different assays for several key plasma biomarkers, including p-tau181 and p-tau217, but additional head-to-head studies are needed, especially for new analytes and for measuring performance in diverse populations. Plasma immunoassays have the potential to increase accessibility of early diagnostic testing for a broad population, including diverse historically under-represented and under-served populations, due to the potential to be implemented globally, including in primary care settings; however, further research is needed to validate the optimal cutoffs for each assay for real-world clinical usage. Eventually, clinical implementation of a two-step workflow may allow standalone use of plasma testing in certain contexts, minimizing the need for confirmation with costly and less accessible cerebrospinal fluid/positron emission tomography testing.
Keywords: Alzheimer's disease; phosphorylated tau assays; plasma immunoassays; tau biomarkers.
© 2024 Janssen Global Services, LLC, a Johsonson & Johnson Company. Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
Rachel Kolster and Gallen Triana‐Baltzer are employees of Janssen Research & Development, LLC. Hartmuth C. Kolb was an employee of Janssen Research & Development, LLC at the time of submission of this paper. Shorena Janelidze is supported by grants from the Swedish Alzheimer Foundation, Stiftelsen för Gamla Tjänarinnor, and Greta och Johan Kocks Stiftelser. Charlotte E. Teunissen has received grants from the European Commission (Marie Curie International Training Network, grant agreement No 860197 [MIRIADE]), JPND, Health∼Holland, the Dutch Research Council (ZonMW), Alzheimer Drug Discovery Foundation, The Selfridges Group Foundation, Alzheimer Netherlands, and the Alzheimer's Association; is an associate editor at
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

