Ultraviolet Photodissociation of the N, N-Dimethylformamide Cation
- PMID: 39625107
- PMCID: PMC11647886
- DOI: 10.1021/acs.jpca.4c06227
Ultraviolet Photodissociation of the N, N-Dimethylformamide Cation
Abstract
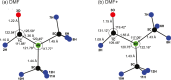
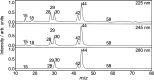
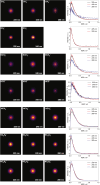
N,N-Dimethylformamide (DMF) provides a useful small-molecule model for studying features of the peptide bond that forms the backbone of proteins. We report results from a comprehensive multimass velocity-map imaging study into the ultraviolet (UV) photolysis of the N,N-dimethylformamide cation (DMF+) at wavelengths of 225, 245, and 280 nm. Electronic structure calculations on DMF and DMF+ were employed to help interpret the experimental results. DMF+ ions are generated by 118 nm single-photon ionization of neutral DMF. Subsequent UV photolysis is found to lead to selective cleavage of the N-CO amide bond. This yields HCO + NC2H6+ as major products, with virtually all of the excess energy released into internal modes of the fragments. The data also indicate a small branching ratio into the HCO+ + NC2H6 product pair, which can be accessed from the 32A' electronic state of DMF+. N-CO bond dissociation can also be accompanied by simultaneous intramolecular hydrogen transfer from the oxygen to the nitrogen end of the amide bond, in which case NCH4+ can be formed efficiently at all three wavelengths. The primary NC2H6+ product is relatively long-lived, but the high degree of internal excitation often results in secondary fragmentation via a variety of pathways to form CH3+, NH4+, NCH2+, and NC2H4+, with secondary dissociation more likely at higher photon energies. The isotropic velocity-map images recorded for the various fragments attest to the long lifetime of NC2H6+ and also imply that dissociation most probably occurs from the same set of electronic states at all wavelengths studied; these are thought to be the 12A' ground state and 22A' first excited state of the DMF+ cation.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Tsaprailis G.; Nair H.; Somogyi A. ´.; Wysocki V. H.; Zhong W.; Futrell J. H.; Summerfield S. G.; Gaskell S. J. J. Am. Chem. Soc. 1999, 121 (22), 5142–5154. 10.1021/ja982980h. - DOI
-
- Biemann K.; Martin S. A. Mass Spectrom. Rev. 1987, 6 (1), 1–75. 10.1002/mas.1280060102. - DOI
-
- Dongré A. R.; Jones J. L.; Somogyi A. ´.; Wysocki V. H. J. Am. Chem. Soc. 1996, 118 (35), 8365–8374. 10.1021/ja9542193. - DOI
LinkOut - more resources
Full Text Sources

