Identification of isoAsp7-Aβ as a major Aβ variant in Alzheimer's disease, dementia with Lewy bodies and vascular dementia
- PMID: 39625512
- PMCID: PMC11615120
- DOI: 10.1007/s00401-024-02824-9
Identification of isoAsp7-Aβ as a major Aβ variant in Alzheimer's disease, dementia with Lewy bodies and vascular dementia
Abstract
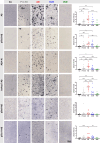
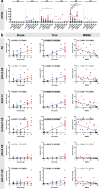
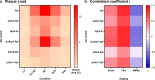
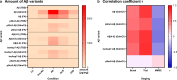
The formation of amyloid-β (Aβ) aggregates in brain is a neuropathological hallmark of Alzheimer's disease (AD). However, there is mounting evidence that Aβ also plays a pathogenic role in other types of dementia and that specific post-translational Aβ modifications contribute to its pathogenic profile. The objective of this study was to test the hypothesis that distinct types of dementia are characterized by specific patterns of post-translationally modified Aβ variants. We conducted a comparative analysis and quantified Aβ as well as Aβ with pyroglutamate (pGlu3-Aβ and pGlu11-Aβ), N-truncation (Aβ(4-X)), isoaspartate racemization (isoAsp7-Aβ and isoAsp27-Aβ), phosphorylation (pSer8-Aβ and pSer26-Aβ) or nitration (3NTyr10-Aβ) modification in post mortem human brain tissue from non-demented control subjects in comparison to tissue classified as pre-symptomatic AD (Pre-AD), AD, dementia with Lewy bodies and vascular dementia. Aβ modification-specific immunohistochemical labelings of brain sections from the posterior superior temporal gyrus were examined by machine learning-based segmentation protocols and immunoassay analyses in brain tissue after sequential Aβ extraction were carried out. Our findings revealed that AD cases displayed the highest concentrations of all Aβ variants followed by dementia with Lewy bodies, Pre-AD, vascular dementia and non-demented controls. With both analytical methods, we identified the isoAsp7-Aβ variant as a highly abundant Aβ form in all clinical conditions, followed by Aβ(4-X), pGlu3-Aβ, pGlu11-Aβ and pSer8-Aβ. These Aβ variants were detected in distinct plaque types of compact, coarse-grained, cored and diffuse morphologies and, with varying frequencies, in cerebral blood vessels. The 3NTyr10-Aβ, pSer26-Aβ and isoAsp27-Aβ variants were not found to be present in Aβ plaques but were detected intraneuronally. There was a strong positive correlation between isoAsp7-Aβ and Thal phase and a moderate negative correlation between isoAsp7-Aβ and performance on the Mini Mental State Examination. Furthermore, the abundance of all Aβ variants was highest in APOE 3/4 carriers. In aggregation assays, the isoAsp7-Aβ, pGlu3-Aβ and pGlu11-Aβ variants showed instant fibril formation without lag phase, whereas Aβ(4-X), pSer26-Aβ and isoAsp27-Aβ did not form fibrils. We conclude that targeting Aβ post-translational modifications, and in particular the highly abundant isoAsp7-Aβ variant, might be considered for diagnostic and therapeutic approaches in different types of dementia. Hence, our findings might have implications for current antibody-based therapies of AD.
Keywords: Abeta; Alzheimer’s disease; Amyloid-β; Automated histology quantification; Dementia with Lewy bodies; Mini Mental State Examination; Post-translational modifications; Vascular dementia.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors declare no competing interests. Consent for publication: All the authors have approved publication.
Figures





References
-
- Alexandru A, Jagla W, Graubner S, Becker A, Bäuscher C, Kohlmann S et al (2011) Selective hippocampal neurodegeneration in transgenic mice expressing small amounts of truncated Aβ is induced by pyroglutamate-Aβ formation. J Neurosci 31:12790–12801. 10.1523/JNEUROSCI.1794-11.2011 - DOI - PMC - PubMed
-
- Arima K, Uéda K, Sunohara N, Arakawa K, Hirai S, Nakamura M et al (1998) NACP/alpha-synuclein immunoreactivity in fibrillary components of neuronal and oligodendroglial cytoplasmic inclusions in the pontine nuclei in multiple system atrophy. Acta Neuropathol 96:439–444. 10.1007/s004010050917 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- SCHI 1437/4-1/German Research Foundation
- WA 1477/6-6/German Research Foundation
- contracts 4001, 0011, 05-901 and 1001/Arizona Biomedical Research Commission
- RO 2226/17-1/German Research Foundation
- P30AG019610 and P30AG072980/AG/NIA NIH HHS/United States
- U24 NS072026/NS/NINDS NIH HHS/United States
- P30 AG019610/AG/NIA NIH HHS/United States
- Fellowship Anna Katharina Kottwitz/Federal State of Saxony-Anhalt
- GRK2824/German Research Foundation
- contract 211002/Arizona Department of Health Services
- P30 AG072980/AG/NIA NIH HHS/United States
- Fellowship Sarah Schrempel/Federal State of Saxony
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

