Vascular smooth muscle cell PRDM16 regulates circadian variation in blood pressure
- PMID: 39625782
- PMCID: PMC11785921
- DOI: 10.1172/JCI183409
Vascular smooth muscle cell PRDM16 regulates circadian variation in blood pressure
Abstract
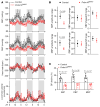
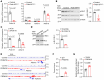
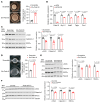
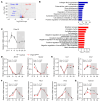
Disruptions of blood pressure (BP) circadian variation are closely associated with an increased risk of cardiovascular disease. Thus, gaining insights into the molecular mechanisms of BP circadian variation is essential for comprehending BP regulation. Human genetic analyses suggest that PR domain-containing protein 16 (PRDM16), a transcription factor highly expressed in vascular smooth muscle cells (VSMCs), is significantly associated with BP-related traits. However, the roles of PRDM16 in BP regulation are largely unknown. Here, we demonstrate that BP in VSMC-specific Prdm16-KO (Prdm16SMKO) mice was significantly lower than that in control mice during the active period, resulting in aberrant BP circadian variation. Mesenteric artery rings from Prdm16SMKO mice showed a reduced response to phenylephrine. Mechanistically, we identified adrenergic receptor α 1d (Adra1d) as a transcriptional target of PRDM16. Notably, PRDM16 exhibited a remarkable circadian expression pattern and regulated the expression of clock genes, particularly Npas2, which is crucial for BP circadian variation regulation. Consequently, PRDM16 deficiency in VSMCs caused disrupted BP circadian variation through a reduced response to adrenergic signaling and clock gene regulation. Our findings provide insights into the intricate molecular pathways that govern circadian fluctuations in BP.
Keywords: Cardiovascular disease; Hypertension; Vascular biology.
Conflict of interest statement
Figures




Comment in
- Blood pressure regulation through circadian variation: PRDM16 as a target in vascular smooth muscle cells doi: 10.1172/JCI188784
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

