Evaluation of the immune status of dogs vaccinated against rabies by an enzyme-linked immunosorbent assay using crude preparations of insect cells infected with a recombinant baculovirus encoding the rabies virus glycoprotein gene
- PMID: 39625902
- PMCID: PMC11614288
- DOI: 10.1371/journal.pone.0314516
Evaluation of the immune status of dogs vaccinated against rabies by an enzyme-linked immunosorbent assay using crude preparations of insect cells infected with a recombinant baculovirus encoding the rabies virus glycoprotein gene
Abstract
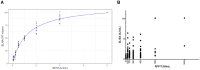
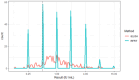
Evaluation of the effectiveness of vaccination of animals against rabies is not routinely implemented. In cases where it is carried out, the rapid fluorescent focus inhibition test (RFFIT) or the fluorescent antibody virus neutralization (FAVN) test are the recommended tests. However, both of these tests require handling of live rabies virus (RABV), and are cumbersome to perform. In view of this, the enzyme-linked immunosorbent assay (ELISA) has been proposed as a surrogate test; however, availability of appropriate antigen is a major impediment for the development of ELISAs to detect anti-rabies antibodies. The most widely used antigen is the RABV glycoprotein (G) purified from cell culture-propagated virus, which requires a biosafety level 3 containment. The alternative is to use recombinantly expressed G, which needs to be to be properly glycosylated and folded to serve as the best antigen. The most suitable system for its production is the baculovirus expression system (BVES). However, purification of RABV G is challenging. We therefore tested partially purified preparations in the form of extracts of insect cells infected with baculovirus expressing RABV G, against sera from vaccinated dogs in an indirect ELISA. The results showed good concordance against RFFIT, with sensitivity and specificity of 90.48% and 80.00%, respectively. The system may be used for quick screening to determine the presence and an approximate level of antibodies, and can be modified to enable monitoring of mass dog vaccination programs, as well as to facilitate certification of dogs intended for international travel and transportation.
Copyright: © 2024 Santosh et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Rupprecht CE, Briggs D, Brown CM, Franka R, Katz SL, Kerr HD, et al. Use of a reduced (4-dose) vaccine schedule for postexposure prophylaxis to prevent human rabies: recommendations of the advisory committee on immunization practices. MMWR Recomm Rep. 2010;59(RR-2):1–9. . - PubMed
-
- WHO. WHO expert consultation on rabies, Third Report. Geneva, Switzerland: World Health Organization, 2018.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

