TRPC6 is a mechanosensitive channel essential for ultrasound neuromodulation in the mammalian brain
- PMID: 39625977
- PMCID: PMC11648612
- DOI: 10.1073/pnas.2404877121
TRPC6 is a mechanosensitive channel essential for ultrasound neuromodulation in the mammalian brain
Abstract
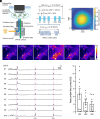
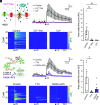
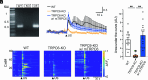
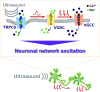
Ultrasound neuromodulation has become an innovative technology that enables noninvasive intervention in mammalian brain circuits with high spatiotemporal precision. Despite the expanding utility of ultrasound neuromodulation in the neuroscience research field and clinical applications, the molecular and cellular mechanisms by which ultrasound impacts neural activity in the brain are still largely unknown. Here, we report that transient receptor potential canonical 6 (TRPC6), a mechanosensitive nonselective cation channel, is essential for ultrasound neuromodulation of mammalian neurons in vitro and in vivo. We first demonstrated that ultrasound irradiation elicited rapid and robust Ca2+ transients mediated via extracellular Ca2+ influx in cultured mouse cortical and hippocampal neurons. Ultrasound-induced neuronal responses were massively diminished by blocking either the generation of action potential or synaptic transmission. Importantly, both pharmacological inhibition and genetic deficiency of TRPC6 almost completely abolished neuronal responses to ultrasound. Furthermore, we found that intracerebroventricular administration of a TRPC6 blocker significantly attenuated the number of neuronal firings in the cerebral cortex evoked by transcranial ultrasound irradiation in mice. Our findings indicate that TRPC6 is an indispensable molecule of ultrasound neuromodulation in intact mammalian brains, providing fundamental understanding of biophysical molecular mechanisms of ultrasound neuromodulation as well as insight into its future feasibility in neuroscience and translational research in humans.
Keywords: TRPC6; cortical neuron; mechanosensitive channels; neuromodulation; ultrasound.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures


References
-
- Temel Y., Jahanshahi A., Neuroscience treating brain disorders with neuromodulation. Science 347, 1418–1419 (2015). - PubMed
-
- Mosilhy E. A., et al. , Non-invasive transcranial brain modulation for neurological disorders treatment: A narrative review. Life Sci. 307, 120869 (2022). - PubMed
-
- Darmani G., et al. , Non-invasive transcranial ultrasound stimulation for neuromodulation. Clin. Neurophysiol. 135, 51–73 (2022). - PubMed
MeSH terms
Substances
Grants and funding
- 18dm0207018/Japan Agency for Medical Research and Development (AMED)
- 19dm0207072/Japan Agency for Medical Research and Development (AMED)
- 20dm0207072/Japan Agency for Medical Research and Development (AMED)
- JPMJMS2024/MEXT | JST | Moonshot Research and Development Program (Moonshot)
- JP21K07580/MEXT | Japan Society for the Promotion of Science (JSPS)
- JP24K02357/MEXT | Japan Society for the Promotion of Science (JSPS)
- JP23K14312/MEXT | Japan Society for the Promotion of Science (JSPS)
- JP23H02790/MEXT | Japan Society for the Promotion of Science (JSPS)
- JP23K18250/MEXT | Japan Society for the Promotion of Science (JSPS)
- n/a/National Institutes for Quantum and Radiological Science and Technology (QST)
- JP24gm6510013/Japan Agency for Medical Research and Development (AMED)
- JP24gm6510015/Japan Agency for Medical Research and Development (AMED)
- JP24zf0127004/Japan Agency for Medical Research and Development (AMED)
- JP21zf0127004/Japan Agency for Medical Research and Development (AMED)
- 22zf0127004s1002/Japan Agency for Medical Research and Development (AMED)
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

