Structural insights into the high basal activity and inverse agonism of the orphan receptor GPR6 implicated in Parkinson's disease
- PMID: 39626010
- PMCID: PMC11850111
- DOI: 10.1126/scisignal.ado8741
Structural insights into the high basal activity and inverse agonism of the orphan receptor GPR6 implicated in Parkinson's disease
Abstract
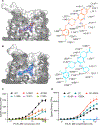
GPR6 is an orphan G protein-coupled receptor with high constitutive activity found in D2-type dopamine receptor-expressing medium spiny neurons of the striatopallidal pathway, which is aberrantly hyperactivated in Parkinson's disease. Here, we solved crystal structures of GPR6 without the addition of a ligand (a pseudo-apo state) and in complex with two inverse agonists, including CVN424, which improved motor symptoms in patients with Parkinson's disease in clinical trials. In addition, we obtained a cryo-electron microscopy structure of the signaling complex between GPR6 and its cognate Gs heterotrimer. The pseudo-apo structure revealed a strong density in the orthosteric pocket of GPR6 corresponding to a lipid-like endogenous ligand. A combination of site-directed mutagenesis, native mass spectrometry, and computer modeling suggested potential mechanisms for high constitutive activity and inverse agonism in GPR6 and identified a series of lipids and ions bound to the receptor. The structures and results obtained in this study could guide the rational design of drugs that modulate GPR6 signaling.
Conflict of interest statement
Figures


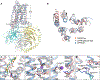


References
-
- Sjöstedt E, Zhong W, Fagerberg L, Karlsson M, Mitsios N, Adori C, Oksvold P, Edfors F, Limiszewska A, Hikmet F, Huang J, Du Y, Lin L, Dong Z, Yang L, Liu X, Jiang H, Xu X, Wang J, Yang H, Bolund L, Mardinoglu A, Zheng C, Von Feilitzen K, Lindskog C, Ponten F, Luo Y, Hokfelt T, Uhlen M, Mulder J, An atlas of the protein-coding genes in the human, pig, and mouse brain. Science 367, eaay5947 (2020). - PubMed
-
- McGregor MM, Nelson AB, Circuit Mechanisms of Parkinson’s Disease. Neuron 101, 1042–1056 (2019). - PubMed
-
- Uhlenbrock K, Gassenhuber H, Kostenis E, Sphingosine 1-phosphate is a ligand of the human gpr3, gpr6 and gpr12 family of constitutively active G protein-coupled receptors. Cell. Signal. 14, 941–953 (2002). - PubMed
-
- Tanaka S, Ishii K, Kasai K, Yoon SO, Saeki Y, Neural expression of G protein-coupled receptors GPR3, GPR6, and GPR12 up-regulates cyclic AMP levels and promotes neurite outgrowth. J. Biol. Chem. 282, 10506–10515 (2007). - PubMed
-
- Padmanabhan S, Myers AG, Prasad BM, Constitutively active GPR6 is located in the intracellular compartments. FEBS Lett. 583, 107–112 (2009). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

