ChREBP-mediated up-regulation of Them1 coordinates thermogenesis with glycolysis and lipogenesis in response to chronic stress
- PMID: 39626011
- PMCID: PMC11817722
- DOI: 10.1126/scisignal.adk7971
ChREBP-mediated up-regulation of Them1 coordinates thermogenesis with glycolysis and lipogenesis in response to chronic stress
Abstract
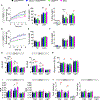
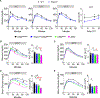
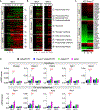
Activation of thermogenic brown adipose tissue (BAT) and inducible beige adipose tissue (BeAT) is triggered by environmental or metabolic stimuli, including cold ambient temperatures and nutrient stress. Thioesterase superfamily member 1 (Them1), a long-chain fatty acyl-CoA thioesterase that is enriched in BAT, suppresses acute cold-induced thermogenesis. Here, we demonstrate that Them1 expression was induced in BAT and BeAT by the carbohydrate response element binding protein (ChREBP) in response to chronic cold exposure or to the activation of the integrated stress response (ISR) by nutrient excess. Under either condition, Them1 suppressed energy expenditure. Consequently, mice lacking Them1 in BAT and BeAT exhibited resistance to obesity and glucose intolerance induced by feeding with a high-fat diet. During chronic cold exposure or ISR activation, Them1 accumulated in the nucleus, where it interacted with ChREBP and reduced the expression of its target genes, including those encoding enzymes that mediate glycolysis and de novo lipogenesis. These findings demonstrate that in response to chronic cold- or nutrient-induced stress, the induction of Them1 by ChREBP limits thermogenesis while coordinately reducing glucose utilization and lipid biosynthesis through its distinct cytoplasmic and nuclear activities. Targeted inhibition of Them1 could be a potential therapeutic approach to increase the activity of BAT and BeAT to enhance energy expenditure in the management of obesity-associated metabolic disorders.
Conflict of interest statement
Figures





References
-
- Cannon B, Nedergaard J, Brown adipose tissue: function and physiological significance. Physiol Rev 84, 277–359 (2004). - PubMed
-
- Loncar D, Convertible adipose tissue in mice. Cell Tissue Res 266, 149–161 (1991). - PubMed
-
- Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, Huang K, Tu H, van Marken Lichtenbelt WD, Hoeks J, Enerback S, Schrauwen P, Spiegelman BM, Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150, 366–376 (2012). - PMC - PubMed
-
- Bartelt A, Heeren J, Adipose tissue browning and metabolic health. Nat Rev Endocrinol 10, 24–36 (2014). - PubMed
-
- Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, Iwanaga T, Miyagawa M, Kameya T, Nakada K, Kawai Y, Tsujisaki M, High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 58, 1526–1531 (2009). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

