Phase II Trial of the PARP Inhibitor, Niraparib, in BAP1 and Other DNA Damage Response Pathway-Deficient Neoplasms
- PMID: 39626160
- PMCID: PMC11616782
- DOI: 10.1200/PO-24-00406
Phase II Trial of the PARP Inhibitor, Niraparib, in BAP1 and Other DNA Damage Response Pathway-Deficient Neoplasms
Erratum in
-
Erratum: Phase II Trial of the PARP Inhibitor, Niraparib, in BAP1 and Other DNA Damage Response Pathway-Deficient Neoplasms.JCO Precis Oncol. 2025 May;9:e2500369. doi: 10.1200/PO-25-00369. Epub 2025 May 22. JCO Precis Oncol. 2025. PMID: 40403212 Free PMC article. No abstract available.
Abstract
Purpose: BRCA1-associated protein 1 (BAP1) is a critical cell cycle and DNA damage response (DDR) regulator with mutations (mBAP1) causing a functional protein loss. PARP inhibitors (PARPis) demonstrate synthetic lethality in mBAP1 preclinical models, independent of underlying BRCA status. This study aimed to explore the clinical activity of niraparib in patients with advanced tumors likely to harbor mBAP1.
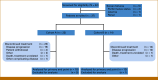
Methods: This was a phase II multicenter trial in which refractory solid tumor patients were assigned to cohort A (histology-specific tumors likely to harbor mBAP1) or cohort B (histology-agnostic tumors with other known non-BRCA-confirmed DDR mutations). All patients received niraparib 300 mg orally once daily on a 28-day cycle. The primary end point was objective response rate, and secondary end points included progression-free survival (PFS) and overall survival.
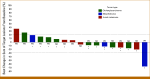
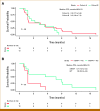
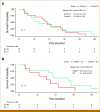
Results: From August 2018 through December 2021, 37 patients were enrolled with 31 evaluable for response (cohort A, n = 18; cohort B, n = 13). In cohort A, the best response was one partial response (PR; 6%), eight stable disease (SD; 44%), and nine progressive disease (PD; 50%). This cohort stopped at the first stage following the prespecified Simon's design. mBAP1 was confirmed in 7/9 patients (78%) with PR or SD but in only 3/9 (33%) in those with PD. The median PFS in patients with mBAP1 (n = 10) was 6.7 months (95% CI, 1.0 to 9.2) versus 1.8 months (95% CI, 0.9 to 4.5) for wild-type (n = 8; P = .020). In cohort B, the best response was six SD (46%) and seven PD (54%), with SD in those with ATM, CHEK2, PTEN, RAD50, and ARID1A mutations.
Conclusion: Niraparib failed to meet the prespecified efficacy end point for response. However, clinical benefit was suggested in a proportion of patients who had a confirmed mBAP1, supporting further investigation.
Trial registration: ClinicalTrials.gov NCT03207347.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures
References
-
- Zabarovsky ER, Lerman MI, Minna JD. Tumor suppressor genes on chromosome 3p involved in the pathogenesis of lung and other cancers. Oncogene. 2002;21:6915–6935. - PubMed
-
- Ismail IH, Davidson R, Gagne JP, et al. Germline mutations in BAP1 impair its function in DNA double-strand break repair. Cancer Res. 2014;74:4282–4294. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

