Congenital T-cell activation impairs transitional-to-follicular B-cell maturation in humans
- PMID: 39626280
- PMCID: PMC11814514
- DOI: 10.1182/bloodadvances.2024013267
Congenital T-cell activation impairs transitional-to-follicular B-cell maturation in humans
Abstract
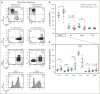
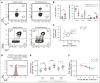
Patients with cytotoxic T-lymphocyte-associated protein 4 (CTLA4) deficiency exhibit profound humoral immune dysfunction, yet the basis for the B-cell defect is not known. We observed a marked reduction in transitional-to-follicular (FO) B-cell development in patients with CTLA4 deficiency, correlating with decreased CTLA4 function in regulatory T cells, increased CD40L levels in effector CD4+ T cells, and increased mammalian target of rapamycin complex 1 (mTORC1) signaling in transitional B cells (TrBs). Treatment of TrBs with CD40L was sufficient to induce mTORC1 signaling and inhibit FO B-cell maturation in vitro. Frequent cell-to-cell contacts between CD40L+ T cells and immunoglobulin D-positive CD27- B cells were observed in patient lymph nodes. FO B-cell maturation in patients with CTLA4 deficiency was partially rescued after CTLA4 replacement therapy in vivo. We conclude that functional regulatory T cells and the containment of excessive T-cell activation may be required for human TrBs to mature and attain metabolic quiescence at the FO B-cell stage.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: J.R.F. was supported by an investigator-initiated research grant from Bristol Myers Squibb. A.K.S. is a founder and serves on the scientific advisory board of Honeycomb Biotechnologies, which is developing Seq-Well arrays for commercial use. S.P. serves on the scientific advisory boards of Abpro Inc, Paratus, and BE Biopharma Inc. H.A.-C. was supported by investigator-initiated research grants from Pfizer, AstraZeneca, Fresenius Kabi, and Boehringer Ingelheim. The remaining authors declare no competing financial interests.
The current affiliation for K.H. is Division of Pediatric Hematology-Oncology, Department of Pediatrics, Hassenfeld Children's Hospital at New York University Langone Health, New York University Grossman School of Medicine, New York, NY.
The current affiliation for J.R.F. is Clinical Immunodeficiency Program of Beth Israel Lahey Health, Division of Allergy and Immunology, Lahey Hospital & Medical Center, Burlington, MA.
Figures






References
-
- Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3(5):541–547. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

