Inaticabtagene autoleucel in adult relapsed or refractory B-cell acute lymphoblastic leukemia
- PMID: 39626300
- PMCID: PMC11872425
- DOI: 10.1182/bloodadvances.2024014182
Inaticabtagene autoleucel in adult relapsed or refractory B-cell acute lymphoblastic leukemia
Abstract
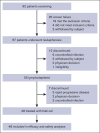
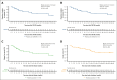
Before November 2023, CD19 chimeric antigen receptor (CAR) T-cell therapies had not been approved in China for patients with relapsed or refractory B-cell acute lymphoblastic leukemia (R/R B-ALL), leaving a significant unmet need. In response, inaticabtagene autoleucel (Inati-cel), a novel CD19 CAR T-cell therapy with a distinct single-chain variable fragment (HI19α), was developed and showed promising efficacy in preliminary clinical research. We conducted a phase 2, single-arm, multicenter study of Inati-cel in adult CD19+ R/R B-ALL in China. The primary end point was the overall remission rate (ORR) at the end of month 3. Forty-eight patients who underwent Inati-cel infusion were evaluated for both efficacy and safety. Among them, 34 patients achieved and maintained remission beyond 3 months, with a 3-month ORR of 70.8% (95% confidence interval [CI], 55.9-83.1). The best ORR was 85.4%, with all responders reaching minimal residual disease negativity. With a median follow-up of 23.7 months, the median duration of remission was 20.7 months (95% CI, 6.4 to not reached), and the median overall survival was not reached (95% CI, 13.0 months to not reached). Additionally, grade ≥3 cytokine release syndrome and neurologic events occurred in 12.5% and 6.2% of patients, respectively. The 2-year follow-up data suggest that Inati-cel demonstrates encouraging and durable responses with manageable safety profiles in R/R B-ALL. Based on the data from this pivotal trial, Inati-cel was approved as the first CAR T-cell therapy for adult R/R B-ALL in China and underscores its potential therapeutic benefits for this patient population. This trial was registered at www.ClinicalTrials.gov as #NCT04684147.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: J.W. reports adviser fees from AbbVie. L.L., J.R., Y.F., Y.Z., and Y.D. own stock option of Juventas Cell Therapy Ltd. The remaining authors declare no competing financial interests.
Figures

References
-
- Pehlivan KC, Duncan BB, Lee DW. CAR-T cell therapy for acute lymphoblastic leukemia: transforming the treatment of relapsed and refractory disease. Curr Hematol Malig Rep. 2018;13(5):396–406. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

