Long-term clinical and safety outcomes from a single-site phase 1 study of neural stem cell transplantation for chronic thoracic spinal cord injury
- PMID: 39626671
- PMCID: PMC11722094
- DOI: 10.1016/j.xcrm.2024.101841
Long-term clinical and safety outcomes from a single-site phase 1 study of neural stem cell transplantation for chronic thoracic spinal cord injury
Abstract
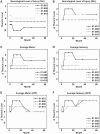
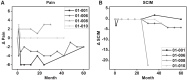
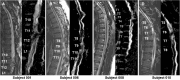
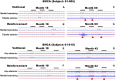
We report the long-term results for a phase 1 study of neural stem cell transplantation for chronic spinal cord injury. The trial was registered on ClinicalTrials.gov as NCT01772810. The primary outcome of the trial was to test the feasibility and safety of human spinal cord-derived neural stem cell (NSI-566) transplantation for the treatment of chronic spinal cord injury in four subjects with thoracic two to thoracic twelve spinal cord injury. Here, we report that all four subjects tolerated the stem cell implantation procedure well, and two subjects had durable electromyography-quantifiable evidence of neurological improvement as well as increased neurological motor and sensory scores at five years post-transplantation.
Keywords: SCI; clinical trial; neural stem cell therapy; neurosurgery; regenerative medicine; spinal cord injury; spinal surgery; stem cells.
Published by Elsevier Inc.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


References
-
- Badhiwala J.H., Ahuja C.S., Fehlings M.G. Time is spine: a review of translational advances in spinal cord injury. J. Neurosurg. Spine. 2019;30:1–18. - PubMed
-
- Abraham M.E., Shalom M., Gendreau J., Gold J., Pierzchajlo G., Pierzchajlo N., Chakravarti S., Sahyouni R., Murthy N., Ciacci J., et al. Utilizing Neuromodulation in the Treatment of Spinal Cord Injury: An Assessment of Clinical Trials from the National ClinicalTrials.gov Database. World Neurosurg. 2023;177:361–367. - PubMed
-
- Curtis E., Martin J.R., Gabel B., Sidhu N., Rzesiewicz T.K., Mandeville R., Van Gorp S., Leerink M., Tadokoro T., Marsala S., et al. A First-in-Human, Phase I Study of Neural Stem Cell Transplantation for Chronic Spinal Cord Injury. Cell Stem Cell. 2018;22:941–950.e6. - PubMed

