Cardiac output-guided haemodynamic therapy for patients undergoing major gastrointestinal surgery: OPTIMISE II randomised clinical trial
- PMID: 39626899
- PMCID: PMC12036648
- DOI: 10.1136/bmj-2024-080439
Cardiac output-guided haemodynamic therapy for patients undergoing major gastrointestinal surgery: OPTIMISE II randomised clinical trial
Abstract
Objectives: To evaluate the clinical effectiveness and safety of a perioperative algorithm for cardiac output-guided haemodynamic therapy in patients undergoing major gastrointestinal surgery.
Design: Multicentre randomised controlled trial.
Setting: Surgical services of 55 hospitals worldwide.
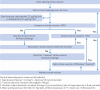
Participants: 2498 adults aged ≥65 years with an American Society of Anesthesiologists physical status classification of II or greater and undergoing major elective gastrointestinal surgery, recruited between January 2017 and September 2022.
Interventions: Participants were assigned to minimally invasive cardiac output-guided intravenous fluid therapy with low dose inotrope infusion during and four hours after surgery, or to usual care without cardiac output monitoring.
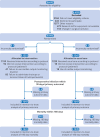
Main outcome measures: The primary outcome was postoperative infection within 30 days of randomisation. Safety outcomes were acute cardiac events within 24 hours and 30 days. Secondary outcomes were acute kidney injury within 30 days and mortality within 180 days.
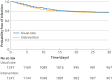
Results: In 2498 patients (mean age 74 (standard deviation 6) years, 57% women), the primary outcome occurred in 289/1247 (23.2%) intervention patients and 283/1247 (22.7%) usual care patients (adjusted odds ratio 1.03 (95% confidence interval 0.84 to 1.25); P=0.81). Acute cardiac events within 24 hours occurred in 38/1250 (3.0%) intervention patients and 21/1247 (1.7%) usual care patients (adjusted odds ratio 1.82 (1.06 to 3.13); P=0.03). This difference was primarily due to an increased incidence of arrhythmias among intervention patients. Acute cardiac events within 30 days occurred in 85/1249 (6.8%) intervention patients and 79/1247 (6.3%) usual care patients (adjusted odds ratio 1.06 (0.77 to 1.47); P=0.71). Other secondary outcomes did not differ.
Conclusions: This clinical effectiveness trial in patients undergoing major elective gastrointestinal surgery did not provide evidence that cardiac output-guided intravenous fluid therapy with low dose inotrope infusion could reduce the incidence of postoperative infections. The intervention was associated with an increased incidence of acute cardiac events within 24 hours, in particular tachyarrhythmias. Based on these findings, the routine use of this treatment approach in unselected patients is not recommended.
Trial registration: ISRCTN Registry ISRCTN39653756.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All writing committee members have completed the ICMJE uniform disclosure form at https://www.icmje.org/disclosure-of-interest/ and declare: this study was funded by Edwards Lifesciences (Irvine, CA, USA) and the UK National Institute for Health and Care Research (NIHR); within the past 36 months, MRE has received research funding from NIHR (grants 15/101/16, 15/80/54, NIHR133554, and NIHR159174); RMP has received consulting fees and honorariums from Edwards Lifesciences (Irvine, CA, USA) and Intersurgical UK; MPG has received unrestricted research funding, consulting fees, and travel costs for participation in a medical advisory board from Edwards Lifesciences, and served on a medical advisory board for SouthWest Sensors (Southampton, UK); MGM was a paid consultant for Edwards Lifesciences until 31 July 2023, has Edwards Lifesciences stock and options, and is an employee of Edwards Lifesciences as of 1 August 2023, shares a patent on a combined intravenous and drinking device, served on a data safety monitoring board for a study of tranexamic acid in major abdominal surgery that was stopped for safety reasons, and is a board member of Evidence Based Perioperative Medicine (EBPOM) Community Interest Company, EBPOM Global (Bridgnorth, UK), Medinspire (Bridgnorth, UK), Mythen Medical (London, UK), and TopMedTalk (Bridgnorth, UK); MAG has received honorariums for lecturing from Edwards Lifesciences; MS has received research grants from Edwards Lifesciences and Medtronic (Dublin, Ireland), licences from Thieme Verlag (Stuttgart, Germany), honorariums from Edwards Lifesciences, BTG (London, UK), and Orion Pharma (UK) (Reading, UK), and has served on a medical advisory board for Edwards Lifesciences; DW has received consulting fees for participation in an advisory board for Edwards Lifesciences, honorariums for lectures from Edwards Lifesciences, and has been a member of an advisory board for Surgery Safety Technologies (Toronto, Canada); SM has participated in an advisory meeting for Edwards Lifesciences; AS has received consulting fees from Edwards Lifesciences and Retia Medical Systems (NY, USA) and has stock or stock options in Retia Medical Systems; JRM has received research funding, consulting fees, honorariums, and support for attending meetings from Edwards Lifesciences, and honorariums and support for attending meetings from Fresenius Kabi (Bad Homburg vor der Höhe, Germany); CH has received payment for expert testimony from FMH (Swiss Medical Association) and has served as president of the Swiss Patient Safety Foundation in Anaesthesia; IG has received honorariums from CSL Vifor (St Gallen, Switzerland), Merck Sharp and Dohme (NJ, USA), Antibiotice (Iasi, Romania), and Ewopharma (Schaffhausen, Switzerland) and has served as chair of the Workforce, Working Conditions, Welfare Standing Committee of the European Board of Anaesthesiology; ST has received institutional research grants from Edwards Lifesciences, Orion Pharma (UK), and Cytosorbents (NJ, USA), and honorariums from Orion Pharma (UK), Amomed Pharma (Vienna, Austria), Cytosorbents, Philips (Amsterdam, Netherlands), and Smith and Nephew (Watford, UK); JVV has served as president of the College of Physicians and Surgeons of Ontario; PR has received research grants from the Canadian Institute for Health Research and the Canadian Society of Anesthesiology; AAM has received support for attending meetings from Edwards Lifesciences and CSL Vifor; and AAG has received consulting fees from Edwards Lifesciences, honorariums from Merck Sharp and Dohme and 3M (Minnesota, USA), and payment for expert testimony from GSK (London, UK), and has served as editor in chief of Revista Española Anestesiología y Reanimación. All other writing committee members declare no competing interests.
Figures

References
-
- Pearse RM, Harrison DA, MacDonald N, et al. OPTIMISE Study Group . Effect of a perioperative, cardiac output-guided hemodynamic therapy algorithm on outcomes following major gastrointestinal surgery: a randomized clinical trial and systematic review. JAMA 2014;311:2181-90. 10.1001/jama.2014.5305 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
