Ubiquitin-Proteasome System in Periodontitis: Mechanisms and Clinical Implications
- PMID: 39626954
- PMCID: PMC11882760
- DOI: 10.1111/cpr.13781
Ubiquitin-Proteasome System in Periodontitis: Mechanisms and Clinical Implications
Abstract
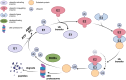
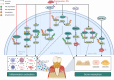
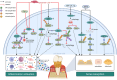
The progression of periodontitis, a bacteria-driven inflammatory and bone-destructive disease, involves myriad cellular and molecular mechanisms. Protein regulation significantly influences the pathogenesis and management of periodontitis. However, research regarding its regulatory role in periodontitis remains relatively limited. The ubiquitin-proteasome system (UPS), which mainly involves ubiquitination by E3 ubiquitin ligases (E3s) and deubiquitination by deubiquitinating enzymes (DUBs), is the primary intracellular and non-lysosomal mechanism of protein degradation. Recent studies have provided compelling evidence to support the involvement of UPS in periodontitis progression. Increasing evidence indicated that E3s, such as CUL3, Nedd4-2, Synoviolin, FBXL19, PDLIM2, TRIMs and TRAFs, modulate inflammatory responses and bone resorption in periodontitis through multiple classical signalling pathways, including NLRP3, GSDMD, NF-κB, Wnt/β-catenin and Nrf2. Meanwhile, DUBs, including OTUD1, A20, CYLD, UCH-L1 and USPs, also broadly modulate periodontitis progression by regulating signalling pathways such as NF-κB, Wnt/β-catenin, NLRP3, and BMP2. Therefore, the modulation of E3s and DUBs has proven to be an effective therapy against periodontitis. This review provides a comprehensive overview of the regulatory role of ubiquitinating and deubiquitinating enzymes in periodontitis progression and the underlying mechanisms. Finally, we summarise several chemical and genetic methods that regulate UPS enzymes and pave the way for the development of targeted therapies for periodontitis.
Keywords: E3 ubiquitin ligases; bone metabolism; deubiquitinating enzymes; inflammation; periodontitis; ubiquitin‐proteasome system.
© 2024 The Author(s). Cell Proliferation published by Beijing Institute for Stem Cell and Regenerative Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Role of E3 ubiquitin ligases and deubiquitinating enzymes in SARS-CoV-2 infection.Front Cell Infect Microbiol. 2023 Jun 9;13:1217383. doi: 10.3389/fcimb.2023.1217383. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37360529 Free PMC article. Review.
-
The E3 ubiquitin ligase MIB2 enhances inflammation by degrading the deubiquitinating enzyme CYLD.J Biol Chem. 2019 Sep 20;294(38):14135-14148. doi: 10.1074/jbc.RA119.010119. Epub 2019 Jul 31. J Biol Chem. 2019. PMID: 31366726 Free PMC article.
-
Ubiquitination and deubiquitination in cancer: from mechanisms to novel therapeutic approaches.Mol Cancer. 2024 Jul 25;23(1):148. doi: 10.1186/s12943-024-02046-3. Mol Cancer. 2024. PMID: 39048965 Free PMC article. Review.
-
E3 ligases and DUBs target ferroptosis: A potential therapeutic strategy for neurodegenerative diseases.Biomed Pharmacother. 2024 Jun;175:116753. doi: 10.1016/j.biopha.2024.116753. Epub 2024 May 17. Biomed Pharmacother. 2024. PMID: 38761423 Review.
-
Hidden targets of ubiquitin proteasome system: To prevent diabetic nephropathy.Pharmacol Res. 2017 Jun;120:170-179. doi: 10.1016/j.phrs.2017.03.024. Epub 2017 Mar 29. Pharmacol Res. 2017. PMID: 28363724 Review.
Cited by
-
Inhibition of the Foxo3/Txnip Axis Alleviates Ventilator-Induced Diaphragmatic Dysfunction by Downregulating MuRF1.Appl Biochem Biotechnol. 2025 Aug;197(8):4949-4968. doi: 10.1007/s12010-025-05261-w. Epub 2025 May 16. Appl Biochem Biotechnol. 2025. PMID: 40377847
-
Mesenchymal stem cells-derived exosomes alleviate liver fibrosis by targeting Hedgehog/SMO signaling.Hepatol Int. 2024 Dec;18(6):1781-1791. doi: 10.1007/s12072-024-10717-y. Epub 2024 Aug 13. Hepatol Int. 2024. PMID: 39138757
References
-
- Zhang X., Wang X., Wu J., et al., “The Global Burden of Periodontal Diseases in 204 Countries and Territories From 1990 to 2019,” Oral Diseases 30, no. 2 (2024): 754–768. - PubMed
-
- Kassebaum N. J., Smith A. G. C., Bernabé E., et al., “Global, Regional, and National Prevalence, Incidence, and Disability‐Adjusted Life Years for Oral Conditions for 195 Countries, 1990‐2015: A Systematic Analysis for the Global Burden of Diseases, Injuries, and Risk Factors,” Journal of Dental Research 96, no. 4 (2017): 380–387. - PMC - PubMed
-
- Genco R. J. and Sanz M., “Clinical and Public Health Implications of Periodontal and Systemic Diseases: An Overview,” Periodontology 2000 83, no. 1 (2020): 7–13. - PubMed
-
- Xu X. W., Liu X., Shi C., and Sun H. C., “Roles of Immune Cells and Mechanisms of Immune Responses in Periodontitis,” Chinese Journal of Dental Research 24, no. 4 (2021): 219–230. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 81901015/National Natural Science Foundation of China
- 82104175/National Natural Science Foundation of China
- LY21H140003/Zhejiang Provincial Natural Science Foundation of China
- Foundation for the new star of the medical industry in Zhejiiang Province
- Y20190102/Wenzhou Municipal Science and Technology Bureau
LinkOut - more resources
Full Text Sources
Miscellaneous

