Boosting Adsorption and Selectivity of Acetylene by Nitro Functionalisation in Copper(II)-Based Metal-Organic Frameworks
- PMID: 39627161
- PMCID: PMC11795735
- DOI: 10.1002/anie.202417183
Boosting Adsorption and Selectivity of Acetylene by Nitro Functionalisation in Copper(II)-Based Metal-Organic Frameworks
Abstract
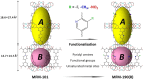
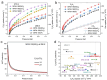
Purification and storage of acetylene (C2H2) are important to many industrial processes. The exploitation of metal-organic framework (MOF) materials to address the balance between selectivity for C2H2 vs carbon dioxide (CO2) against maximising uptake of C2H2 has attracted much interest. Herein, we report that the synergy between unsaturated Cu(II) sites and functional groups, fluoro (-F), methyl (-CH3), nitro (-NO2) in a series of isostructural MOF materials MFM-190(R) that show exceptional adsorption and selectivity of C2H2. At 298 K, MFM-190(NO2) exhibits an C2H2 uptake of 216 cm3 g-1 (320 cm3 g-1 at 273 K) at 1.0 bar and a high selectivity for C2H2/CO2 (up to ~150 for v/v = 2/1) relevant to that in the industrial cracking stream. Dynamic breakthrough studies validate and confirm the excellent separation of C2H2/CO2 by MFM-190(NO2) under ambient conditions. In situ neutron powder diffraction reveals the cooperative binding, packing and selectivity of C2H2 by unsaturated Cu(II) sites and free -NO2 groups.
© 2024 The Author(s). Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Zhang L., Jiang K., Yang L. F., Li L. B., Hu E. L., Yang L., Shao K., Xing H. B., Cui Y. J., Yang Y., Li B., Chen B. L., Qian G. D., Angew. Chem. Int. Ed. 2021, 60, 15995–16002. - PubMed
-
- Wang L. Y., Sun W. Q., Zhang Y. B., Xu N., Krishna R., Hu J. B., Jiang Y. J., He Y. B., Xing H. B., Angew. Chem. Int. Ed. 2021, 60, 22865–22870. - PubMed
-
- Pei J. Y., Wen H. M., Gu X. W., Qian Q. L., Yang Y., Cui Y. J., Li B., Chen B. L., Qian G. D., Angew. Chem. Int. Ed. 2021, 60, 25068–25074. - PubMed
-
- Han X., Yang S., Angew. Chem. Int. Ed. 2023, 135, e202218274. - PubMed
-
- Zhang Z. Q., Peh S. B., Krishna R., Kang C. J., Chai K. G., Wang Y. X., Shi D. C., Zhao D., Angew. Chem. Int. Ed. 2021, 60, 17198–17204. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

