Multiple insular-prefrontal pathways underlie perception to execution during response inhibition in humans
- PMID: 39627197
- PMCID: PMC11615282
- DOI: 10.1038/s41467-024-54564-9
Multiple insular-prefrontal pathways underlie perception to execution during response inhibition in humans
Abstract
Inhibiting prepotent responses in the face of external stop signals requires complex information processing, from perceptual to control processing. However, the cerebral circuits underlying these processes remain elusive. In this study, we used neuroimaging and brain stimulation to investigate the interplay between human brain regions during response inhibition at the whole-brain level. Magnetic resonance imaging suggested a sequential four-step processing pathway: initiating from the primary visual cortex (V1), progressing to the dorsal anterior insula (daINS), then involving two essential regions in the inferior frontal cortex (IFC), namely the ventral posterior IFC (vpIFC) and anterior IFC (aIFC), and reaching the basal ganglia (BG)/primary motor cortex (M1). A combination of ultrasound stimulation and time-resolved magnetic stimulation elucidated the causal influence of daINS on vpIFC and the unidirectional dependence of aIFC on vpIFC. These results unveil asymmetric pathways in the insular-prefrontal cortex and outline the macroscopic cerebral circuits for response inhibition: V1→daINS→vpIFC/aIFC→BG/M1.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures
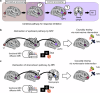
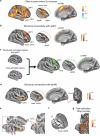

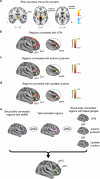



References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

