Biodegradable Janus sonozyme with continuous reactive oxygen species regulation for treating infected critical-sized bone defects
- PMID: 39627239
- PMCID: PMC11615367
- DOI: 10.1038/s41467-024-54894-8
Biodegradable Janus sonozyme with continuous reactive oxygen species regulation for treating infected critical-sized bone defects
Abstract
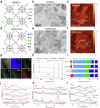
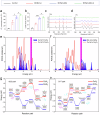
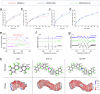
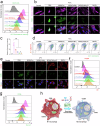
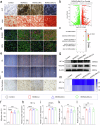
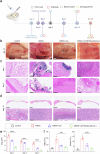
Critical-sized bone defects are usually accompanied by bacterial infection leading to inflammation and bone nonunion. However, existing biodegradable materials lack long-term therapeutical effect because of their gradual degradation. Here, a degradable material with continuous ROS modulation is proposed, defined as a sonozyme due to its functions as a sonosensitizer and a nanoenzyme. Before degradation, the sonozyme can exert an effective sonodynamic antimicrobial effect through the dual active sites of MnN4 and Cu2O8. Furthermore, it can promote anti-inflammation by superoxide dismutase- and catalase-like activities. Following degradation, quercetin-metal chelation exhibits a sustaining antioxidant effect through ligand-metal charge transfer, while the released ions and quercetin also have great self-antimicrobial, osteogenic, and angiogenic effects. A rat model of infected cranial defects demonstrates the sonozyme can rapidly eliminate bacteria and promote bone regeneration. This work presents a promising approach to engineer biodegradable materials with long-time effects for infectious bone defects.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



References
-
- Pearson, J. J. et al. In vivo hydroxyapatite scaffold performance in infected bone defects. J. Biomed. Mater. Res. B Appl. Biomater.108, 1157–1166 (2020). - PubMed
-
- Lei, C. et al. Heterostructured piezocatalytic nanoparticles with enhanced ultrasound response for efficient repair of infectious bone defects. Acta Biomater.172, 343–354 (2023). - PubMed
-
- Lei, J. et al. Sulfur-regulated defect engineering for enhanced ultrasonic piezocatalytic therapy of bacteria-infected bone defects. Chem. Eng. J.435, 14 (2022).
-
- Feng, X. et al. Piezo-augmented sonosensitizer with strong ultrasound-propelling ability for efficient treatment of osteomyelitis. ACS Nano16, 2546–2557 (2022). - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
- 82272459/National Natural Science Foundation of China (National Science Foundation of China)
- 82372380/National Natural Science Foundation of China (National Science Foundation of China)
- 22175058/National Natural Science Foundation of China (National Science Foundation of China)
- 82302763/National Natural Science Foundation of China (National Science Foundation of China)
- 2023AFB770/Natural Science Foundation of Hubei Province (Hubei Provincial Natural Science Foundation)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

