Assessment of molecular modulation by multifrequency electromagnetic pulses to preferably eradicate tumorigenic cells
- PMID: 39627265
- PMCID: PMC11615363
- DOI: 10.1038/s41598-024-81171-x
Assessment of molecular modulation by multifrequency electromagnetic pulses to preferably eradicate tumorigenic cells
Abstract
Physics methods of cancer therapy are extensively used in clinical practice, but they are invasive and often confront undesired side effects. A fully new equipment that allows sustained emission of intense and time-controlled non-ionizing multifrequency electromagnetic pulse (MEMP), has been applied to eukaryotic cells in culture. The equipment discriminates the overall electronegative charge of the cell cultures, and its subsequent proportional emission may thereby become higher and lethal to cancer cells of generally high metabolic activity. In contrast, low tumorigenic cells would be much less affected. We tested the specificity and efficacy of the equipment against a collection of (i) highly tumorigenic cells of human (glioblastoma, cervical carcinoma, and skin) and mouse (colon adenocarcinoma) origin; (ii) cell lines of much lower tumorigenicity (non-human primate kidney and mouse fibroblasts), and (iii) primary porcine macrophages lacking tumorigenicity. Time and intensity control of the MEMP allowed progressive decay of viability fairly correlating to cell tumorigenicity, which was provoked by a proportional alteration of the cytoplasmic membrane permeability, cell cycle arrest at G2, and general collapse of the actin cytoskeleton to the perinuclear region. Correspondingly, these effects drastically inhibited the proliferative capacity of the most tumorigenic cells in clonogenic assays. Moreover, MEMP suppressed in a dose-dependent manner the tumorigenicity of retrovirally transduced luciferase expressing colon adenocarcinoma cells in xenografted immune-competent mice, as determined by tumor growth in a bioluminescence imaging system. Our results support MEMP as an anti-cancer non-invasive physical treatment of substantial specificity for tumorigenic cells with promising therapeutic potential in oncology.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures
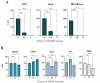



 ). The profiles were obtained by FACS Calibur flow cytometry (BD Science), and cell cycle progression was based on DNA quantification. Cells were modulated for 5 min, then collected at 48 h afterward, fixed and stained with the PI/RNase buffer (BD Pharmingen).
). The profiles were obtained by FACS Calibur flow cytometry (BD Science), and cell cycle progression was based on DNA quantification. Cells were modulated for 5 min, then collected at 48 h afterward, fixed and stained with the PI/RNase buffer (BD Pharmingen).

References
-
- Berg, H. et al. Bioelectromagnetic field effects on cancer cells and mice tumors. Electromagn. Biol. Med.29, 132–143. 10.3109/15368371003776725 (2010). - PubMed
-
- Williams, C. D., Markov, M. S., Hardman, W. E. & Cameron, I. L. Therapeutic electromagnetic field effects on angiogenesis and tumor growth. Anticancer Res.21, 3887–3891 (2001). - PubMed
-
- Yamaguchi, S., Ogiue-Ikeda, M., Sekino, M. & Ueno, S. Effects of pulsed magnetic stimulation on tumor development and immune functions in mice. Bioelectromagnetics27, 64–72. 10.1002/bem.20177 (2006). - PubMed
-
- Koh, E. K. et al. A 60-Hz sinusoidal magnetic field induces apoptosis of prostate cancer cells through reactive oxygen species. Int. J. Radiat. Biol.84, 945–955. 10.1080/09553000802460206 (2008). - PubMed
-
- Li, J., Ma, Y., Li, N., Cao, Y. & Zhu, Y. Natural static magnetic field-induced apoptosis in liver cancer cell. Electromagn. Biol. Med.33, 47–50. 10.3109/15368378.2013.783850 (2014). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

