Effects of the therapeutic correction of U1 snRNP complex on Alzheimer's disease
- PMID: 39627450
- PMCID: PMC11615310
- DOI: 10.1038/s41598-024-81687-2
Effects of the therapeutic correction of U1 snRNP complex on Alzheimer's disease
Abstract
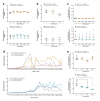
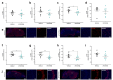
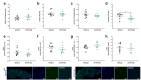
The U1 snRNP complex recognizes pre-mRNA splicing sites in the early stages of spliceosome assembly and suppresses premature cleavage and polyadenylation. Its dysfunction may precede Alzheimer's disease (AD) hallmarks. Here we evaluated the effects of a synthetic single-stranded cDNA (APT20TTMG) that interacts with U1 snRNP, in iPSC-derived neurons from a donor diagnosed with AD and in the SAMP8 mouse model. APT20TTMG effectively binds to U1 snRNP, specifically decreasing TAU in AD neurons, without changing mitochondrial activity or glutamate. Treatment enhanced neuronal electrical activity, promoted an enrichment of differentially expressed genes related to key processes affected by AD. In SAMP8 mice, APT20TTMG reduced insoluble pTAU in the hippocampus, amyloid-beta and GFAP in the cortex, and U1-70 K in both brain regions, without cognitive changes. This study highlights the correction of the U1 snRNP complex as a new target for AD.
Keywords: Alzheimer’s disease; Amyloid-beta; Astrogliosis; TAU; U1 snRNP; U1-70K.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: C.B.Q.S.L. has filed a patent related to this work (US11946050B2). C.G.M.Z., V.V.C.S., and E.S.S. are employees of Aptah Bio Inc. C.B.Q.S.L. and R.M.B. are co-founders of Aptah Bio Inc. R.M.B. is an employee of Vesper Biotechnologies. B.P., A.C.W., X.Y.C., and R.V.S. are employees of Ananda Devices Inc. M.H.M. is a founder of Ananda Devices Inc. M.S.R. and L.B. are members of the scientific advisory board of Aptah Bio Inc.
Figures




References
-
- Cheng, Z., Shang, Y., Gao, S. & Zhang, T. Overexpression of U1 snRNA induces decrease of U1 spliceosome function associated with Alzheimer’s disease. J. Neurogenet.31, 337–343 (2017). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

