Placental nanoparticle-mediated IGF1 gene therapy corrects fetal growth restriction in a guinea pig model
- PMID: 39627510
- PMCID: PMC12105984
- DOI: 10.1038/s41434-024-00508-3
Placental nanoparticle-mediated IGF1 gene therapy corrects fetal growth restriction in a guinea pig model
Abstract
Fetal growth restriction (FGR) caused by placental insufficiency is a major contributor to neonatal morbidity and mortality. There is currently no in utero treatment for placental insufficiency or FGR. The placenta serves as the vital communication, supply, exchange, and defense organ for the developing fetus and offers an excellent opportunity for therapeutic interventions. Here we show efficacy of repeated treatments of trophoblast-specific human insulin-like 1 growth factor (IGF1) gene therapy delivered in a non-viral, polymer nanoparticle to the placenta for the treatment of FGR. Using a guinea pig maternal nutrient restriction model (70% food intake) of FGR, nanoparticle-mediated IGF1 treatment was delivered to the placenta via ultrasound guidance across the second half of pregnancy, after establishment of FGR. This treatment resulted in correction of fetal weight in MNR + IGF1 animals compared to sham treated controls on an ad libitum diet, increased fetal blood glucose and decreased fetal blood cortisol levels compared to sham treated MNR, and showed no negative maternal side-effects. Overall, we show a therapy capable of positively impacting the entire pregnancy environment: maternal, placental, and fetal. This combined with our previous studies using this therapy at mid pregnancy in the guinea pig and in two different mouse model and three different human in vitro/ex vivo models, demonstrate the plausibility of this therapy for future human translation. Our overall goal is to improve health outcomes of neonates and decrease numerous morbidities associated with the developmental origins of disease.
© 2024. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approval: Animal care and usage was approved by the University of Florida Intuitional Animal Care and Usage Committee (Protocol #202011236).
Figures


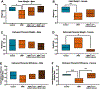

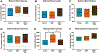
Update of
-
Placental Nanoparticle-mediated IGF1 Gene Therapy Corrects Fetal Growth Restriction in a Guinea Pig Model.bioRxiv [Preprint]. 2024 Sep 21:2024.04.05.587765. doi: 10.1101/2024.04.05.587765. bioRxiv. 2024. Update in: Gene Ther. 2025 May;32(3):255-265. doi: 10.1038/s41434-024-00508-3. PMID: 38645174 Free PMC article. Updated. Preprint.
Similar articles
-
Mid-Pregnancy Placental Transcriptome in a Model of Placental Insufficiency with and without Novel Intervention.Reprod Sci. 2025 Feb;32(2):435-443. doi: 10.1007/s43032-024-01769-4. Epub 2024 Dec 20. Reprod Sci. 2025. PMID: 39707140 Free PMC article.
-
Placental Nanoparticle-mediated IGF1 Gene Therapy Corrects Fetal Growth Restriction in a Guinea Pig Model.bioRxiv [Preprint]. 2024 Sep 21:2024.04.05.587765. doi: 10.1101/2024.04.05.587765. bioRxiv. 2024. Update in: Gene Ther. 2025 May;32(3):255-265. doi: 10.1038/s41434-024-00508-3. PMID: 38645174 Free PMC article. Updated. Preprint.
-
Maternal-fetal interfaces transcriptome changes associated with placental insufficiency and a novel gene therapy intervention.Physiol Genomics. 2025 Jan 1;57(1):8-15. doi: 10.1152/physiolgenomics.00131.2024. Epub 2024 Oct 7. Physiol Genomics. 2025. PMID: 39374081 Free PMC article.
-
Adaptations of the human placenta to hypoxia: opportunities for interventions in fetal growth restriction.Hum Reprod Update. 2021 Apr 21;27(3):531-569. doi: 10.1093/humupd/dmaa053. Hum Reprod Update. 2021. PMID: 33377492 Review.
-
Placenta-directed gene therapy for fetal growth restriction.Semin Fetal Neonatal Med. 2017 Dec;22(6):415-422. doi: 10.1016/j.siny.2017.04.005. Epub 2017 May 15. Semin Fetal Neonatal Med. 2017. PMID: 28522033 Review.
Cited by
-
Placenta hIGF1 nanoparticle treatment in guinea pigs mitigates FGR-associated fetal sex-dependent effects on liver metabolism-related signaling pathways.Am J Physiol Endocrinol Metab. 2025 Mar 1;328(3):E395-E409. doi: 10.1152/ajpendo.00440.2024. Epub 2025 Feb 5. Am J Physiol Endocrinol Metab. 2025. PMID: 39907801 Free PMC article.
-
Mid-Pregnancy Placental Transcriptome in a Model of Placental Insufficiency with and without Novel Intervention.Reprod Sci. 2025 Feb;32(2):435-443. doi: 10.1007/s43032-024-01769-4. Epub 2024 Dec 20. Reprod Sci. 2025. PMID: 39707140 Free PMC article.
References
-
- Keenan L. 1 in 10 babies worldwide are born early, with major impacts on health and survival. World Health Organization, 2023.
-
- Audette MC, Kingdom JC. Screening for fetal growth restriction and placental insufficiency. Semin Fetal Neonatal Med. 2018;23:119–25. - PubMed
-
- Nardozza LM, Caetano AC, Zamarian AC, Mazzola JB, Silva CP, Marçal VM, et al. Fetal growth restriction: current knowledge. Arch Gynecol Obstet. 2017;295:1061–77. - PubMed
-
- de Onis M, Blössner M. Villar J, Levels and patterns of intrauterine growth retardation in developing countries. Eur J Clin Nutr. 1998;52Suppl 1:S5–15. - PubMed
-
- Malacova E, Regan A, Nassar N, Raynes-Greenow C, Leonard H, Srinivasjois R, et al. Risk of stillbirth, preterm delivery, and fetal growth restriction following exposure in a previous birth: systematic review and meta-analysis. BJOG. 2018;125:183–92. - PubMed
MeSH terms
Substances
Grants and funding
- K99 HD109458/HD/NICHD NIH HHS/United States
- R01 HD090657/HD/NICHD NIH HHS/United States
- K99HD109458/U.S. Department of Health & Human Services | NIH | Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)
- R01HD090657/U.S. Department of Health & Human Services | NIH | Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

