Blood-based biomarkers for early frailty are sex-specific: validation of a combined in silico prediction and data-driven approach
- PMID: 39627572
- PMCID: PMC12181598
- DOI: 10.1007/s11357-024-01449-w
Blood-based biomarkers for early frailty are sex-specific: validation of a combined in silico prediction and data-driven approach
Abstract
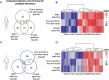
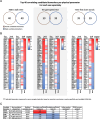
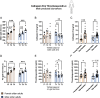
Frailty is characterized by loss of physical function and is preferably diagnosed at an early stage (e.g., during pre-frailty). Unfortunately, sensitive tools that can aid early detection are lacking. Blood-based biomarkers, reflecting pathophysiological adaptations before physical symptoms become apparent, could be such tools. We identified candidate biomarkers using a mechanism-based computational approach which integrates a priori defined database-derived clinical biomarkers and skeletal muscle transcriptome data. Identified candidate biomarkers were used as input for a sex-specific correlation analysis, using individual gene expression data from female (n = 24) and male (n = 28) older adults (all 75 + years, ranging from fit to pre-frail) and three frailty-related physical parameters. Male and female groups were matched based on age, BMI, and Fried frailty index. The best correlating candidate biomarkers were evaluated, and selected biomarkers were measured in serum. In females, myostatin and galectin-1 and, in males, cathepsin B and thrombospondin-4 serum levels were significantly different between the physically weakest and fittest participants (all p < 0.05). Logistic regression confirmed the added value of these biomarkers in conjunction with age and BMI to predict whether the subjects belonged to the weaker or fittest group (AUC = 0.80 in females and AUC = 0.83 in males). In conclusion, both in silico and in vivo analyses revealed the sex-specificity of candidate biomarkers, and we identified a selection of potential biomarkers which could be used in a biomarker panel for early detection of frailty. Further investigation is needed to confirm these leads for early detection of frailty.
Keywords: Biomarker identification; Circulating markers; Diagnosis; Monitoring; Prevention; Screening.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures
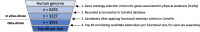



References
-
- Gordon EH, Peel NM, Samanta M, Theou O, Howlett SE, Hubbard RE. Sex differences in frailty: a systematic review and meta-analysis. Exp Gerontol. 2017;89:30–40. 10.1016/j.exger.2016.12.021. - PubMed
-
- O’Caoimh R, Sezgin D, O’Donovan MR, William Molloy D, Clegg A, Rockwood K, Liew A. Prevalence of frailty in 62 countries across the world: a systematic review and meta-analysis of population-level studies. Age Ageing. 2021;50:96–104. 10.1093/ageing/afaa219. - PubMed
-
- Kojima G, Iliffe S, Jivraj S, Walters K. Association between frailty and quality of life among community-dwelling older people: a systematic review and meta-analysis. J Epidemiol Commun Health. 2016;70:716–21. 10.1136/jech-2015-206717. - PubMed
-
- Hanlon P, Nicholl BI, Jani BD, Lee D, McQueenie R, Mair FS. Frailty and pre-frailty in middle-aged and older adults and its association with multimorbidity and mortality: a prospective analysis of 493 737 UK Biobank participants. Lancet Public Heal. 2018;3:e323–32. 10.1016/S2468-2667(18)30091-4. - PMC - PubMed
-
- Abizanda P, Romero L, Sánchez-jurado PM, Martínez-reig M, Gómez-arnedo L, Alfonso SA. Frailty and mortality, disability and mobility loss in a Spanish cohort of older adults: the FRADEA Study. Maturitas. 2013;74:54–60. 10.1016/j.maturitas.2012.09.018. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

