Uterine sarcoma with KAT6B/A::KANSL1 fusion: a molecular and clinicopathological study on 9 cases
- PMID: 39627614
- PMCID: PMC11950137
- DOI: 10.1007/s00428-024-03994-3
Uterine sarcoma with KAT6B/A::KANSL1 fusion: a molecular and clinicopathological study on 9 cases
Abstract
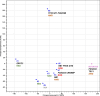
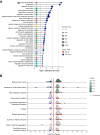
Uterine sarcomas with KAT6B/A::KANSL1 fusion represent a new entity characterized by bland morphology, commonly with hybrid features of low-grade endometrial stromal sarcoma (LG-ESS) and tumors with smooth muscle differentiation. In our study, we performed a detailed morphological, immunohistochemical, and molecular analysis of 9 cases of these tumors. Six of those had been originally diagnosed as LG-ESS, one as leiomyoma, one as leiomyosarcoma, and the remaining case as sarcoma with the KAT6B/A::KANSL1 fusion. Seven cases showed overlapping features between endometrial stromal and smooth muscle tumors, one case resembled cellular leiomyoma, and one case resembled high-grade endometrial stromal sarcoma. Immunohistochemically, the tumors showed a common expression of smooth muscle markers and endometrial stromal markers. Molecular findings showed the KAT6B/A::KANSL1 fusion in all cases (by NGS and FISH). In addition, mutations affecting genes such as TP53, PDGFRB, NF1, RB1, PTEN, ATM, RB1, FANCD2, and TSC1 were present in all 5 cases with aggressive behavior. One patient with no evidence of disease showed no additional mutations, while another harbored a mutation of a single gene (ERCC3). Of the 8 patients with available follow-up, two died of disease, 3 are currently alive with disease, and 3 have no evidence of disease. The correct recognition of tumors with the KAT6B/A::KANSL1 fusion is essential because despite the bland morphological features of most cases, these tumors have a propensity for aggressive behavior.
Keywords: Endometrial stromal sarcoma; KAT6B/A::KANSL1 fusion; Next generation sequencing; Uterine tumor.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval: The study has been approved by the Ethics Committee of General University Hospital in Prague in compliance with the Helsinki Declaration (No. 2140/19 S-IV). The Ethics Committee waived the requirement for informed consent, as according to the Czech Law (Act. no. 373/11, and its amendment Act no. 202/17), it is not necessary to obtain informed consent in fully anonymized studies. Conflict of interest: The authors declare no competing interests.
Figures


References
-
- Dundr P, Matej R, Hojny J, Hajkova N, Nemejcova K, Kendall Bartu M (2024) The spectrum of fusions occurring in non-smooth muscle mesenchymal uterine tumors: a review of the current knowledge. Arch Pathol Lab Med Mar 15, Online ahead of print. 10.5858/arpa.2023-0324-RA - PubMed
-
- Parra-Herran C, Howitt BE (2019) Uterine mesenchymal tumors: update on classification, staging, and molecular features. Surg Pathol Clin 12(2):363–396. 10.1016/j.path.2019.01.004 - PubMed
-
- Croce S, Devouassoux-Shisheboran M, Pautier P et al (2022) Uterine sarcomas and rare uterine mesenchymal tumors with malignant potential. Diagnostic guidelines of the french sarcoma group and the rare gynecological tumors group. Gynecol Oncol 167(2):373–389. 10.1016/j.ygyno.2022.07.031 - PubMed
-
- Parra-Herran C, Schoolmeester JK, Yuan L et al (2016) Myxoid leiomyosarcoma of the uterus: a clinicopathologic analysis of 30 cases and review of the literature with reappraisal of its distinction from other uterine myxoid mesenchymal neoplasms. Am J Surg Pathol 40(3):285–301. 10.1097/PAS.0000000000000593 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

