Impact of the digital health application ViViRA on spinal mobility, physical function, quality of life and pain perception in spondyloarthritides patients: a randomized controlled trial
- PMID: 39627851
- PMCID: PMC11613898
- DOI: 10.1186/s13075-024-03443-1
Impact of the digital health application ViViRA on spinal mobility, physical function, quality of life and pain perception in spondyloarthritides patients: a randomized controlled trial
Abstract
Background: Spondyloarthritides (SpAs) are a group of common rheumatic diseases that often cause limited mobility and lower back pain. Physiotherapy is an integral part of treatment, but access to physiotherapy limits treatment success. Digital health applications (DHAs) enable home-based physiotherapy and could significantly improve access for SpAs patients. The aim is to investigate the clinical effects of the DHA ViViRA compared with those of standard physiotherapy.
Methods: SpAs patients with chronic back pain were enrolled in a randomized controlled trial. The intervention group received ViViRA DHA, whereas the control group received standard physiotherapy. Pain (verbal rating scale, PAIN-Detect), quality of life (SF-36) and mobility (BASMI) were assessed at baseline and after 12 weeks as the primary outcomes.
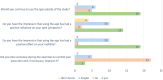
Results: Data from 59 participants (71.2% female, mean age 45.2 years) were analyzed. The intervention group showed a significant improvement in mobility (average BASMI score: baseline: 1.1 [range 0.7-1.5]; follow-up: 1.0 [range 0.5-1.4]; p = 0.05), whereas the control group showed a significant decrease in mobility (baseline: 1.5 [range 1.1-1.9]; follow-up: 1.8 [range 1.4-2.2]; p = 0.00). The intervention group demonstrated lower pain intensity (VRS pain level at week 3.5 ± 2.8) than did the control group (VRS pain level at week 4.5 ± 2) after 12 weeks.
Conclusion: Our results highlight the efficacy of DHAs such as ViViRA in the treatment of lower back pain in SpAs patients. Compared with the current gold standard, physiotherapy, DHA use results in superior outcomes. However, further larger studies are needed to confirm these promising results.
Trial registration: The study is registered in the German clinical trial registry (DRKS) under the following ID: DRKS00031254.
Keywords: DHA; Digital health application; E-Health; Mobility; Physical function; Psoriatic spondylitis; Spondyloarthritides; Spondyloarthropathy; ViViRA.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study protocol was approved by the medical faculty ethics committee of the Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany (22-425-Bm) and registered in the German Clinical Trials Register (DRKS-ID DRKS00031254). Participation in the study was voluntary. All patients gave their written informed consent before study inclusion. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


References
-
- Albrecht K, Binder S, Minden K, Poddubnyy D, Regierer AC, Strangfeld A, Callhoff J. Systematisches Review Zur Schätzung Der Prävalenz entzündlich Rheumatischer Erkrankungen in Deutschland [Systematic review to estimate the prevalence of inflammatory rheumatic diseases in Germany. German Version] Z Rheumatol. 2023;82(9):727–38. 10.1007/s00393-022-01305-2. German. - PMC - PubMed
-
- van den Berg R, van der Heijde DM. How should we diagnose spondyloarthritis according to the ASAS classification criteria: a guide for practicing physicians. Pol Arch Med Wewn. 2010;120(11):452–7. PMID: 21102381. - PubMed
-
- Feldtkeller E, Khan MA, van der Heijde D, van der Linden S, Braun J. Age at disease onset and diagnosis delay in HLA-B27 negative vs. positive patients with ankylosing spondylitis. Rheumatol Int. 2003;23(2):61 – 6. 10.1007/s00296-002-0237-4. Epub 2002 Sep 3. PMID: 12634937. - PubMed
-
- Senbekov M, Saliev T, Bukeyeva Z, Almabayeva A, Zhanaliyeva M, Aitenova N, Toishibekov Y, Fakhradiyev I. The recent progress and applications of Digital Technologies in Healthcare: a review. Int J Telemed Appl. 2020;2020:8830200. 10.1155/2020/8830200. PMID: 33343657; PMCID: PMC7732404. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

