The chemoprotective effect of anti-platelet agents on cancer incidence in people with non-alcoholic fatty liver disease (NAFLD): a retrospective cohort study
- PMID: 39627877
- PMCID: PMC11613771
- DOI: 10.1186/s12916-024-03802-4
The chemoprotective effect of anti-platelet agents on cancer incidence in people with non-alcoholic fatty liver disease (NAFLD): a retrospective cohort study
Erratum in
-
Correction: The chemoprotective effect of anti-platelet agents on cancer incidence in people with non-alcoholic fatty liver disease (NAFLD): a retrospective cohort study.BMC Med. 2025 Mar 31;23(1):193. doi: 10.1186/s12916-025-04033-x. BMC Med. 2025. PMID: 40165209 Free PMC article. No abstract available.
Abstract
Background: Non-alcoholic fatty liver disease (NAFLD) is associated with an increased incidence of hepatic and extrahepatic cancers, in particular those linked to obesity. In people with chronic liver disease, aspirin may confer protection against hepatocellular carcinoma (HCC). We explore the potential chemoprotective effect of aspirin/other anti-platelet agents on obesity-related cancers, including HCC in people with NAFLD.
Methods: We performed a retrospective cohort study of anonymised electronic medical records using the TriNetX network (Cambridge, MA, USA), a global federated database. We identified adults aged 18 or over with a diagnosis of NAFLD, prior to commencing antiplatelet agents. Two groups were created: antiplatelet (1) versus no antiplatelet use (2). We propensity score matched for nine variables. Antiplatelet use was defined as aspirin, ticagrelor, cangrelor, clopidogrel or prasugrel use for at least 1 year. The outcomes of interest were incidence of HCC and other obesity-related cancers. Follow-up was for 5 years. We performed subgroup analyses on aspirin users only and stratified findings for sex and age. Sensitivity analysis was conducted on individuals with 3- and 5-year aspirin exposure.
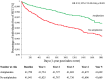
Results: Post matching, there were 42,192 people per group. Antiplatelet use in people with NAFLD was associated with statistically significant reduction in all obesity-related cancers (HR 0.71, 95% CI 0.65-0.78, p < 0.001) and individually for HCC (HR 0.52, 95% CI 0.40-0.68, p < 0.001), breast carcinoma (HR 0.78, 95% CI 0.66-0.92, p = 0.003), pancreatic carcinoma (HR 0.61, 95% CI 0.47-0.78, p < 0.001) and colorectal carcinoma (HR 0.68, 95% CI 0.56-0.84, p < 0.001). For women, there was a significant reduction in risk of ovarian carcinoma (HR 0.75, 95% CI 0.57-0.98, p = 0.034). Aspirin monotherapy was similarly associated with reduced incidence of HCC (HR 0.46, 95% CI 0.32-0.64, p < 0.001) and all obesity-related cancers (HR 0.71, 95% CI, 0.56-0.90, p = 0.004), with benefits observed in males (HR 0.71, 95% CI 0.56-0.90, p = 0.004), females (HR 0.77, 95% CI 0.67-0.88, p < 0.001) and in older (HR 0.72, 95% CI 0.63-0.82, p < 0.001) but not younger people (HR 0.78, 95% CI 0.60-1.03, p = 0.589).
Conclusions: Aspirin/antiplatelet agents may have a role in primary cancer prevention in people living with NAFLD.
Keywords: Anti-platelets; Aspirin; Hepatocellular carcinoma; Non-alcoholic fatty liver disease; Obesity.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: MA receives funding and salary contribution from the Novo Nordisk UK research foundation. DJC has received investigator-initiated grants from Astra Zeneca and Novo Nordisk and support for education from Perspectum. GHI is an employee of TriNetX LLC. UA has received honoraria from Eli Lilly, Procter & Gamble, Viatris, Grunenthal and Sanofi for educational meetings and funding for attendance to an educational meeting from Diiachi Sankyo. UA has also received investigator-led funding by Procter & Gamble and is a council member of the Royal Society of Medicine’s Vascular, Lipid & Metabolic Medicine Section.
Figures



References
-
- WHO European Regional Obesity Report 2022. Copenhagen: WHO Regional Office for Europe; 2022. p. 1.
-
- Riazi K, Azhari H, Charette JH, et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2022;7:851–61. - PubMed
-
- Paik JM, Kabbara K, Eberly KE, et al. Global burden of NAFLD and chronic liver disease among adolescents and young adults. Hepatology. 2022;75:1204–17. - PubMed
-
- Targher G, Byrne CD, Lonardo A, et al. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta-analysis. J Hepatol. 2016;65:589–600. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

