Exosomes derived from minor salivary gland mesenchymal stem cells: a promising novel exosome exhibiting pro-angiogenic and wound healing effects similar to those of adipose-derived stem cell exosomes
- PMID: 39627883
- PMCID: PMC11616330
- DOI: 10.1186/s13287-024-04069-5
Exosomes derived from minor salivary gland mesenchymal stem cells: a promising novel exosome exhibiting pro-angiogenic and wound healing effects similar to those of adipose-derived stem cell exosomes
Abstract
Backgrounds: Minor salivary gland mesenchymal stem cells (MSGMSCs) can be easily extracted and have a broad range of sources. Applying exosomes to wounds is a highly promising method for promoting wound healing. Exosomes derived from different stem cell types have been proven to enhance wound healing, with adipose-derived stem cell (ADSC)-derived exosomes being the most extensively researched. Considering that MSGMSCs have advantages such as easier extraction compared to ADSCs, MSGMSCs should also be a very promising type of stem cell in exosome therapy. However, whether MSGMSC-derived exosomes (MSGMSC-exos) can promote wound healing and how they compare to ADSC-derived exosomes (ADSC-exos) in the wound healing process remain unclear.
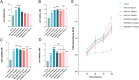
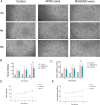
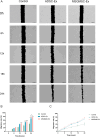
Materials: The effects of MSGMSC-exos and ADSC-exos on angiogenesis in wound healing were investigated in vitro using CCK-8, scratch assays, and tube formation assays. Subsequently, the promotion of wound healing by MSGMSC-exos and ADSC-exos was evaluated in vivo using a full-thickness wound defect model in mice. Immunohistochemistry was used to verify the effects of MSGMSC-exos and ADSC-exos on promoting collagen deposition, angiogenesis, and cell proliferation in the wound. Immunofluorescence staining was performed to investigate the role of MSGMSC-exos and ADSC-exos in modulating the inflammatory response in the wound. Furthermore, proteomic sequencing was conducted to investigate the functional similarities and differences between the proteomes of MSGMSC-exos and ADSC-exos, with key protein contents verified by ELISA.
Results: MSGMSC-exos exhibited similar effects as ADSC-exos in promoting the migration, proliferation, and tube formation of human umbilical vein endothelial cells (HUVECs) in vitro, with a comparable dose-dependent effect. In vivo experiments confirmed that MSGMSC-exos have similar wound healing-promoting functions as ADSC-exos. MSGMSC-exos promoted the neovascularization and maturation of blood vessels in vivo at a level comparable to ADSC-exos. Despite MSGMSC-exos showing less collagen deposition than ADSC-exos, they exhibited stronger anti-scar formation and anti-inflammatory effects. Proteomic analysis revealed that the proteins promoting wound healing in both MSGMSC-exos and ADSC-exos were relatively conserved, with ITGB1 identified as a critical protein for angiogenesis. Further differential analysis revealed that the functions specifically enriched in MSGMSC-exos and ADSC-exos reflected the functions of their source tissue.
Conclusions: Our study confirms that MSGMSC-exos exhibit highly similar wound healing and angiogenesis-promoting functions compared to ADSC-exos, and the proteins involved in promoting wound healing in both are relatively conserved. Moreover, MSGMSC-exos show stronger anti-scar formation and anti-inflammatory effects than ADSC-exos. This suggests that MSGMSCs are a promising stem cell source with broad applications in wound healing treatment.
Keywords: Adipose-derived stem cells; Exosome therapy; Human minor salivary mesenchymal stem cells; Wound healing.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The extraction procedures of human MSGMSCs and ADSCs were conducted in accordance with the Declaration of Helsinki, and informed consent was obtained from the donors and/or their guardians before the tooth collection. The animal experiments were conducted following the ARRIVE guidelines 2.0 (Animal Research: Reporting of In Vivo Experiments). The extraction procedures of human MSGMSCs and all animal experiments in this study were approved by the Ethics Committee of Peking University Third Hospital (Project title: Research on the tissue engineering and regenerative medicine application of human minor salivary mesenchymal stem cells No: S2019144, Date of approval: December 19, 2019). The extraction procedures of human ADSCs in this study was approved by the Ethics Committee of Peking University Third Hospital (Project title: Basic research of the application of adipose tissue in the field of plastic surgery No: LM2020365, Date of approval: October 21, 2020). Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures






References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

