Utilizing an Opportunistic Clinical Study and Population-Based Pharmacokinetic Models to Identify Rational Empiric Dosing Regimens for Piperacillin-Tazobactam in Critically Ill Patients
- PMID: 39628093
- PMCID: PMC11938006
- DOI: 10.1002/jcph.6161
Utilizing an Opportunistic Clinical Study and Population-Based Pharmacokinetic Models to Identify Rational Empiric Dosing Regimens for Piperacillin-Tazobactam in Critically Ill Patients
Abstract
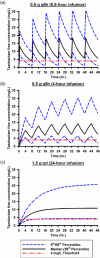
Determining an effective dosing regimen for piperacillin-tazobactam in critically ill patients is challenging due to substantial pharmacokinetic variability caused by complex pathophysiological changes. To address this need, a prospective clinical study was conducted, which enrolled 112 critically ill patients and employed an opportunistic sampling strategy. Population modeling and simulation were performed to characterize the pharmacokinetics (PK) and probability of target attainment (PTA) of piperacillin-tazobactam under various dosing regimens. Both piperacillin and tazobactam final models were one-compartment models with zero-order input and first-order elimination. Significant covariates included lean body weight for piperacillin and creatinine clearance along with continuous renal replacement therapy (CRRT) for both drugs. Monte Carlo simulations demonstrated that continuous infusion can achieve higher PTA than intermittent and extended infusions. When considering the minimum inhibitory concentration (MIC) of 16 mg/L for Pseudomonas aeruginosa (a frequently encountered bacterial pathogen among critically ill patients) and a PK/PD target of 100% fT >MIC, continuous infusion of 6 g/day is recommended for critically ill patients with a CLcr <60 mL/min, 9 g/day for patients with CLcr in the range of 60 to 129 mL/min, and 12 g/day for patients with a CLcr ≥130 mL/min. In addition, extended infusion represents a good alternative, especially the 3 g q6h or 4 g q6h regimens which can achieve the designated European Committee on Antimicrobial Susceptibility Testing (EUCAST) non-species-related PK/PD breakpoint of 8 mg/L. Our study provided valuable insight into PTA outcomes, which, together with individual renal function of future patients and institution-specific piperacillin susceptibility patterns, may assist physicians when making dosing decisions.
Keywords: empiric antibiotic dosing regimen selection; opportunistic clinical study; pharmacometric modeling; piperacillin–tazobactam; population pharmacokinetics.
© 2024 The Author(s). The Journal of Clinical Pharmacology published by Wiley Periodicals LLC on behalf of American College of Clinical Pharmacology.
Conflict of interest statement
None.
Figures



References
-
- Roberts JA, Paul SK, Akova M, et al. DALI: defining antibiotic levels in intensive care unit patients: are current β‐lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis. 2014;58(8):1072‐1083. - PubMed
-
- McKinnon PS, Paladino JA, Schentag JJ. Evaluation of area under the inhibitory curve (AUIC) and time above the minimum inhibitory concentration (T>MIC) as predictors of outcome for cefepime and ceftazidime in serious bacterial infections. Int J Antimicrob Agents. 2008;31(4):345‐351. - PubMed
-
- Pfizer . ZOSYN (piperacillin and tazobactam) for injection. FDA. Accessed October 19, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/050684s88s89s9...
-
- Sorgel F, Kinzig M. Pharmacokinetic characteristics of piperacillin/tazobactam. Intensive Care Med. 1994;20(Suppl 3):S14‐S20. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

