Extensive influence of microsporidian infection on sucrose solution consumption, antioxidant enzyme activity, cell structure, and lifespan of Asian honeybees
- PMID: 39628478
- PMCID: PMC11611804
- DOI: 10.3389/fimmu.2024.1404766
Extensive influence of microsporidian infection on sucrose solution consumption, antioxidant enzyme activity, cell structure, and lifespan of Asian honeybees
Abstract
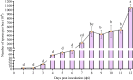
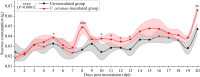
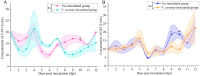
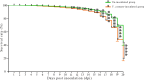
Apis cerana is the original host of Vairimorpha (Nosema) ceranae, a widespread fungal parasite that causes bee nosemosis, which severely threatens the health of bee colonies and the sustainable development of the apiculture industry. To evaluate the impact of V. ceranae infection on A. c. cerana workers, V. ceranae spores were purified and used to inoculate newly emerged workers to evaluate the effects of V. ceranae infection. This was followed by an in-depth investigation of V. ceranae spore load and host sucrose solution consumption. Activities of four major antioxidant enzymes (SOD, PPO, CAT, and GST) were determined. Paraffin sections of the host midgut tissue were prepared and subjected to microscopic observation. The survival rates of V. ceranae-inoculated and uninoculated workers were analyzed. The results showed that spore load gradually increased and peaked at 12 dpi. The consumption of workers in the V. ceranae-inoculated group was extremely significant higher (P < 0.0001) than that of workers in the un-inoculated group. The results of antioxidant enzyme activity were suggestive of positive host defense via catalase (CAT) and glutathione-S-transferase (GST) in the middle stage of infection, as well as the negative fungal impact on superoxide dismutase (SOD) and polyphenol oxidase (PPO) at the whole stage of infection, reflecting the complex host-parasite interaction. Additionally, we observed a disruption in the structure of the host midgut epithelial cells. Moreover, the survival rate of workers in V. ceranae-inoculated groups was nearly always lower than that of workers in the uninoculated groups. These results demonstrate a consistent increase in spore load with the proliferation of V. ceranae, leading to persistent energetic stress and midgut epithelial cell structural damage to the host, ultimately resulting in a shortened lifespan for the host. Our findings enhance the current understanding of the interactions between A. cerana and V. ceranae as well as provide a solid basis for exploring the mechanisms underlying host response and V. ceranae infection.
Keywords: Apis cerana; Vairimorpha (Nosema) ceranae; antioxidant enzyme; honeybee; host-parasite interaction.
Copyright © 2024 Fan, Zhao, Zang, Dong, Qiu, Song, Li, Jiang, Wu, Lü, Zhou, Fu, Chen and Guo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Impact of Nosema ceranae and Nosema apis on individual worker bees of the two host species (Apis cerana and Apis mellifera) and regulation of host immune response.J Insect Physiol. 2018 Feb-Mar;105:1-8. doi: 10.1016/j.jinsphys.2017.12.010. Epub 2017 Dec 29. J Insect Physiol. 2018. PMID: 29289505
-
In-depth investigation of microRNA-mediated cross-kingdom regulation between Asian honey bee and microsporidian.Front Microbiol. 2022 Sep 29;13:1003294. doi: 10.3389/fmicb.2022.1003294. eCollection 2022. Front Microbiol. 2022. PMID: 36246221 Free PMC article.
-
Comparative Transcriptome Investigation of Nosema ceranae Infecting Eastern Honey Bee Workers.Insects. 2022 Feb 28;13(3):241. doi: 10.3390/insects13030241. Insects. 2022. PMID: 35323539 Free PMC article.
-
Nosema ceranae in European honey bees (Apis mellifera).J Invertebr Pathol. 2010 Jan;103 Suppl 1:S73-9. doi: 10.1016/j.jip.2009.06.017. Epub 2009 Nov 11. J Invertebr Pathol. 2010. PMID: 19909977 Review.
-
Pathogen- and host-directed pharmacologic strategies for control of Vairimorpha (Nosema) spp. infection in honey bees.J Eukaryot Microbiol. 2024 Sep-Oct;71(5):e13026. doi: 10.1111/jeu.13026. Epub 2024 Apr 4. J Eukaryot Microbiol. 2024. PMID: 38572630 Review.
Cited by
-
Microsporidian infection of mosquito larvae changes the host-associated microbiome towards the synthesis of antimicrobial factors.Parasit Vectors. 2025 May 17;18(1):178. doi: 10.1186/s13071-025-06813-z. Parasit Vectors. 2025. PMID: 40382661 Free PMC article.
-
Analysis of the Expression Patterns of piRNAs in Response to Microsporidian Invasion in Midgut of Workers (Apis cerana cerana).Int J Mol Sci. 2025 Mar 7;26(6):2402. doi: 10.3390/ijms26062402. Int J Mol Sci. 2025. PMID: 40141043 Free PMC article.
References
-
- Fries I, Feng F, da Silva AD, Slemenda SB, Pieniazek NJ. Nosema ceranae n. sp.(Microspora, Nosematidae), morphological and molecular characterization of a microsporidian parasite of the Asian honey bee Apis cerana (Hymenoptera, Apidae). Eur J Protistol. (1996) 32:356–65. doi: 10.1016/S0932-4739(96)80059-9 - DOI
-
- Boncristiani H, Ellis JD, Bustamante T, Graham J, Jack C, Kimmel C, et al. . World honey bee health: the global distribution of western honey bee (Apis mellifera L.) pests and pathogens. Bee World. (2020) 98:1–5. doi: 10.1080/0005772X.2020.180033 - DOI
-
- Paxton R. Does infection by Nosema ceranae cause “Colony Collapse Disorder” in honey bees (Apis mellifera)? J Apicultural Res. (2010) 49:80–84. doi: 10.3896/IBRA.1.49.1.11 - DOI
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

