Alpha-1-antitrypsin as novel substrate for S. aureus' Spl proteases - implications for virulence
- PMID: 39628483
- PMCID: PMC11611844
- DOI: 10.3389/fimmu.2024.1481181
Alpha-1-antitrypsin as novel substrate for S. aureus' Spl proteases - implications for virulence
Abstract
Background: The serine protease like (Spl) proteases of Staphylococcus aureus are a family of six proteases whose function and impact on virulence are poorly understood. Here we propose alpha-1-antitrypsin (AAT), an important immunomodulatory serine protease inhibitor as target of SplD, E and F. AAT is an acute phase protein, interacting with many proteases and crucial for prevention of excess tissue damage by neutrophil elastase during the innate immune response to infections.
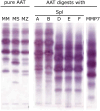
Methods: We used MALDI-TOF-MS to identify the cleavage site of Spl proteases within AAT's reactive center loop (RCL) and LC-MS/MS to quantify the resulting peptide cleavage product in in vitro digestions of AAT and heterologous expressed proteases or culture supernatants from different S. aureus strains. We further confirmed proteolytic cleavage and formation of a covalent complex with Western Blots, investigated AAT's inhibitory potential against Spls and examined the NETosis inhibitory activity of AAT-Spl-digestions.
Results: SplD, E and F, but not A or B, cleave AAT in its RCL, resulting in the release of a peptide consisting of AAT's C-terminal 36 amino acids (C36). Synthetic C36, as well as AAT-SplD/E/F-digestions exhibit NETosis inhibition. Only SplE, but not D or F, was partly inhibited by AAT, forming a covalent complex.
Conclusion: We unraveled a new virulence trait of S. aureus, where SplD/E/F cleave and inactivate AAT while the cleavage product C36 inhibits NETosis.
Keywords: AAT; C-terminal Alpha-1-Antitrypsin peptides; CAAPs; NETosis; Staphylococcus aureus; host-pathogen interaction; virulence.
Copyright © 2024 Scherr, Darisipudi, Börner, Austermeier, Hoffmann, Eberhardt, Abdurrahman, Saade, von Eggeling, Kasper, Holtfreter, Bröker and Kiehntopf.
Conflict of interest statement
MK is an inventor of a patent application covering the utilized LC-MS/MS method as a tool for characterizing systemic inflammation applicant: Jena University Hospital; inventors: Arite Bigalke and MK; published as EP4224163A1. Jena University Hospital is owner of a patent related to methods determining the origin of an infection EP3239712: granted; inventors: MK, Diana Schmerler. A patent covering the initial identification of C42 was granted as well published as CN104204808B, JP6308946B2, US10712350B2, EP2592421B1, EP2780719B1; owner: Jena University Hospital; Inventors: MK, Diana Schmerler, Thomas Deufel, Frank Brunkhorst. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Weiner LM, Webb AK, Limbago B, Dudeck MA, Patel J, Kallen AJ, et al. . Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the national healthcare safety network at the centers for disease control and prevention, 2011-2014. Infect Control Hosp Epidemiol. (2016) 37:1288–301. doi: 10.1017/ice.2016.174 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

