Dietary caloric restriction protects experimental autoimmune uveitis by regulating Teff/Treg balance
- PMID: 39628557
- PMCID: PMC11612795
- DOI: 10.1016/j.isci.2024.111279
Dietary caloric restriction protects experimental autoimmune uveitis by regulating Teff/Treg balance
Abstract
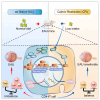
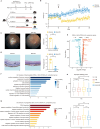
Uveitis, an autoimmune disease, often leads to blindness. CD4+ T cells, including regulatory T cells (Tregs) and effector T cells (Th1 and Th17), play a critical role in its pathogenesis. Caloric restriction (CR) has been shown to alleviate autoimmune diseases. However, careful characterization of the impact of CR on experimental autoimmune uveitis (EAU) is poorly understood. This study used single-cell RNA sequencing to analyze cervical draining lymph nodes in mice under ad libitum (AL) and CR diets, with or without EAU. CR increased Tregs, altered immune cell metabolism, reduced EAU symptoms, and downregulated inflammatory and glycolysis genes. Flow cytometry confirmed CR's inhibitory effect on Th1 and Th17 proliferation and its promotion of Treg proliferation. CR also balanced CD4+ T cells by inhibiting the PI3K/AKT/c-Myc pathway and reducing GM-CSF in Th17 cells. These findings suggest CR as a potential therapeutic strategy for autoimmune diseases.
Keywords: Diet; Immune response; Transcriptomics.
© 2024 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
Beneficial mechanisms of dimethyl fumarate in autoimmune uveitis: insights from single-cell RNA sequencing.J Neuroinflammation. 2024 Apr 29;21(1):112. doi: 10.1186/s12974-024-03096-6. J Neuroinflammation. 2024. PMID: 38684986 Free PMC article.
-
Activation of the Notch signaling pathway disturbs the CD4+/CD8+, Th17/Treg balance in rats with experimental autoimmune uveitis.Inflamm Res. 2019 Sep;68(9):761-774. doi: 10.1007/s00011-019-01260-w. Epub 2019 Jun 17. Inflamm Res. 2019. PMID: 31209505
-
Longdan Xiegan Decoction alleviates experimental autoimmune uveitis in rats by inhibiting Notch signaling pathway activation and Th17 cell differentiation.Biomed Pharmacother. 2021 Apr;136:111291. doi: 10.1016/j.biopha.2021.111291. Epub 2021 Jan 22. Biomed Pharmacother. 2021. PMID: 33493870
-
CD4+ T-Cell Plasticity in Non-Infectious Retinal Inflammatory Disease.Int J Mol Sci. 2021 Sep 3;22(17):9584. doi: 10.3390/ijms22179584. Int J Mol Sci. 2021. PMID: 34502490 Free PMC article. Review.
-
Green tea EGCG, T cells, and T cell-mediated autoimmune diseases.Mol Aspects Med. 2012 Feb;33(1):107-18. doi: 10.1016/j.mam.2011.10.001. Epub 2011 Oct 14. Mol Aspects Med. 2012. PMID: 22020144 Review.
Cited by
-
From Dysbiosis to Disease: The Microbiome's Influence on Uveitis Pathogenesis.Microorganisms. 2025 Jan 25;13(2):271. doi: 10.3390/microorganisms13020271. Microorganisms. 2025. PMID: 40005638 Free PMC article. Review.
-
Gut microbiome dysregulation in noninfectious uveitis.Front Immunol. 2025 Jul 29;16:1614304. doi: 10.3389/fimmu.2025.1614304. eCollection 2025. Front Immunol. 2025. PMID: 40799652 Free PMC article. Review.
References
-
- Chong W.P., Horai R., Mattapallil M.J., Silver P.B., Chen J., Zhou R., Sergeev Y., Villasmil R., Chan C.C., Caspi R.R. IL-27p28 inhibits central nervous system autoimmunity by concurrently antagonizing Th1 and Th17 responses. J. Autoimmun. 2014;50:12–22. doi: 10.1016/j.jaut.2013.08.003. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

