Autophagy in reproduction and pregnancy-associated diseases
- PMID: 39628569
- PMCID: PMC11613427
- DOI: 10.1016/j.isci.2024.111268
Autophagy in reproduction and pregnancy-associated diseases
Abstract
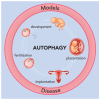
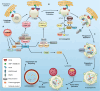
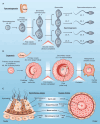
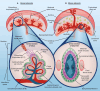
As advantageous as sexual reproduction is during progeny generation, it is also an expensive and treacherous reproductive strategy. The viviparous eukaryote has evolved to survive stress before, during, and after pregnancy. An important and conserved intracellular pathway for the control of metabolic stress is autophagy. The autophagy process occurs in multiple stages through the coordinated action of autophagy-related genes. This review summarizes the evidence that autophagy is an integral component of reproduction. Additionally, we discuss emerging in vitro techniques that will enable cellular and molecular studies of autophagy and its associated pathways in reproduction. Finally, we discuss the role of autophagy in the pathogenesis and progression of several pregnancy-related disorders such as preterm birth, preeclampsia, and intra-uterine growth restriction, and its potential as a therapeutic target.
Keywords: cell biology; molecular biology; pathophysiology; physiology.
Conflict of interest statement
All authors declare no competing interests.
Figures
References
-
- Michod R.E., Levin B.R. The evolution of sex: an examination of current ideas. Sinauer Associates, Incorporated; 1988.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

