Age-related changes in pupil dynamics and task modulation across the healthy lifespan
- PMID: 39628657
- PMCID: PMC11611812
- DOI: 10.3389/fnins.2024.1445727
Age-related changes in pupil dynamics and task modulation across the healthy lifespan
Abstract
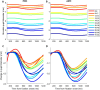
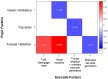
The pupil is modulated by luminance, arousal, bottom-up sensory, and top-down cognitive signals, and has increasingly been used to assess these aspects of brain functioning in health and disease. However, changes in pupil dynamics across the lifespan have not been extensively examined, hindering our ability to fully utilize the pupil in probing these underlying neural processes in development and aging in healthy and clinical cohorts. Here, we examined pupil responses during the interleaved pro-/anti-saccade task (IPAST) in healthy participants across the lifespan (n = 567, 5-93 years of age). Based on the extracted measurements of pupil dynamics, we demonstrated age-related changes in pupil measures and task modulation. Moreover, we characterized the underlying factors and age-related effects in components of pupil responses that may be attributed to developmental and aging changes in the associated brain regions. Finally, correlations between factors of pupil dynamics and saccade behaviors revealed evidence of shared neural processes in the pupil and saccade control circuitries. Together, these results demonstrate changes in pupil dynamics as a result of development and aging, providing a baseline with which altered pupil responses due to neurological deficits at different ages can be studied.
Keywords: aging; anti-saccade; development; pupillary response; saccade preparation.
Copyright © 2024 Huang, Smorenburg, Yep, Riek, Calancie, Kirkpatrick, Brien, Coe, Wang and Munoz.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
LinkOut - more resources
Full Text Sources

