Research Integrity in Guidelines and evIDence synthesis (RIGID): a framework for assessing research integrity in guideline development and evidence synthesis
- PMID: 39628711
- PMCID: PMC11612463
- DOI: 10.1016/j.eclinm.2024.102717
Research Integrity in Guidelines and evIDence synthesis (RIGID): a framework for assessing research integrity in guideline development and evidence synthesis
Abstract
Background: Clinical guidelines rely on sound evidence to underpin recommendations for patient care. Compromised research integrity can erode public trust and the credibility of the scientific enterprise, with potential harm to patients. Despite increased recognition of integrity concerns in scientific literature, there are no processes or guidance for incorporating integrity assessments into evidence-based guideline development or evidence synthesis more broadly.
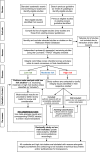
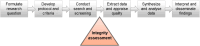
Methods: In response to this crucial gap, we developed the Research Integrity in Guidelines and evIDence synthesis (RIGID) framework. Co-developed with international input from 80 multidisciplinary experts, and consumers, the RIGID framework and accompanying checklist provide an innovative and transparent six-step approach to assess the integrity of studies during the synthesis of evidence, including in the development of clinical guidelines. Central to the framework is an integrity committee, responsible for objective assessments and allocations, with constructive author engagement.
Findings: The six key steps of the RIGID framework are described, as follows: (1) Review: standard systematic review processes are followed, in line with approved evidence synthesis methodologies; (2) Exclude: studies which have been retracted are excluded, and those with expressions of concern are flagged for further evaluation; (3) Assess: remaining studies are assessed for integrity using an appropriate tool and allocated an initial integrity risk rating of low, moderate or high risk for integrity concerns; (4) Discuss: integrity assessment results are discussed among integrity committee members with votes to determine final integrity risk rating allocations for each study; (5) Establish contact: low risk studies are included without author contact, whereas authors of studies ranked as moderate or high risk are contacted for clarification; (6) Reassess: studies are reassessed for inclusion using the RIGID author response algorithm (reclassified as 'included' where authors have provided a satisfactory response, 'awaiting classification' where authors have engaged but time is needed to address concerns, or 'not included' where authors have not responded to contact attempts). An illustrative case study is presented, where these six steps of the RIGID framework were successfully implemented in an influential international guideline endorsed by 39 societies across six continents. Following implementation of the framework, 45 of the 101 originally identified studies (45%) were not included in the guideline.
Interpretation: Based on the latest literature and international expertise, the RIGID framework represents an important advancement in best practice standards for guideline development and evidence synthesis. Using this resource, guideline developers, policy-makers, clinicians and scientists are better positioned to navigate the currently precarious research landscape to ensure evidence synthesis and subsequent clinical recommendations prioritize patient care and preserve the sanctity of scientific endeavors.
Funding: This study received no specific funding. The guideline in which it was piloted was supported by the Australian National Health and Medical Research Council (NHMRC) for guideline development through the Centre of Research Excellence (CRE) in Women's Health in Reproductive Life (CRE-WHiRL) (APP1171592) and the CRE in Polycystic Ovary Syndrome (CRE-PCOS) (APP1078444) led by Monash University, Australia, and partner societies: the American Society for Reproductive Medicine (ASRM), the US Endocrine Society (ENDO), the European Society of Endocrinology (ESE) and the European Society of Human Reproduction and Embryology (ESHRE).
Keywords: Clinical practice recommendations; Evidence synthesis; Evidence-based guidelines; Guideline development; Research integrity; Retraction.
© 2024 The Author(s).
Conflict of interest statement
All authors have completed the Unified Competing Interest form (available on request from the corresponding author) and declare support from the Australian National Health and Medical Research Council (NHMRC) for guideline development through the Centre for Research Excellence (CRE) in Women's Health in Reproductive Life (CRE-WHiRL) (APP1171592) and the CRE in Polycystic Ovary Syndrome (CRE-PCOS) (APP1078444) led by Monash University, Australia, and partner societies: the American Society for Reproductive Medicine (ASRM), the US Endocrine Society (ENDO), the European Society of Endocrinology (ESE) and the European Society of Human Reproduction and Embryology (ESHRE). The primary funders of the guideline, NHMRC, were not involved in development of the guideline or the RIGID framework and have not influenced the scope. They set standards for guideline development and, based on independent peer review, approve the guideline process. AM, RW and WL are supported by fellowships from the NHMRC. CTT receives salary support from the NHMRC-funded CRE-WHiRL and chairs their early career group, and has previously chaired the Androgen-Excess in PCOS society (AE-PCOS) early career group (2020–2022). BWM received a fellowship from the NHMRC; research funding from Merck KGaA; consulting fees for consultancy for ObsEva and Merck; and travel funding support from Merck. RN is on the Editorial committee for the Fertility and Sterility journal, chairs the committee at Westmead fertility and has a mentoring role at Flinders Fertility; and has received grant funding from the NHMRC; consulting fees from VinMec Vietnam; payment or honoraria from Cadilla Pharma for lectures; and travel support funding from Merck to speak at meetings. HT is an Executive of the International Society of Endocrinology, received a fellowship from the NHMRC, was the recipient of the NHMRC CRE-WHiRL grant and CRE-PCOS grant funding the guideline development and receives travel funding support from professional societies to attend and give plenary and symposium lectures as an independent academic and clinical expert. None of the listed organizations had any role in the design, conduct, preparation or decision to publish this manuscript.
Figures


References
-
- Steneck N.H. U.S. Department of Health and Human Services; USA: 2007. Office of research integrity (ORI): introduction to the responsible conduct of research.
-
- Bordewijk E.M., Wang R., Askie L.M., et al. Data integrity of 35 randomised controlled trials in women' health. Eur J Obstet Gynecol Reprod Biol. 2020;249:72–83. - PubMed
-
- COPE COPE Promoting integrity in research and its publication. 2023. https://publicationethics.org/
LinkOut - more resources
Full Text Sources
Miscellaneous

